Preparation, Administration, and Handling of Chemotherapy
The chemotherapy administration process provides many opportunities for various health care professionals to make mistakes. The error may occur anywhere in the chemotherapy administration system, from the moment the oncologist writes the chemotherapy order to the actual drug administration by the nurse. A breakdown in this multistep process can cause lethal consequences. When the error is not detected before it reaches the patient, the patient suffers the serious and toxic effects. In December 1999, the Institute of Medicine reported that 44,000 to 98,000 Americans die each year from preventable errors (LNN, Dec. 6, p. 190). A study by Edgar et al. (1994) found that of the 43 medication errors that resulted in death, 11 involved chemotherapy overdoses. The celebrated case of the Boston Globe reporter who died of a chemotherapy error in 1994 was a wake-up call for health care institutions to invest more time and effort in examining their medication administration system. Diligent analysis found that in most institutions, system failures were the primary cause of medication errors. Since then, system-based changes and guidelines have been implemented. Voluntary, rather than mandatory, error reports have risen dramatically because “no one
feared punishment for honestly reporting mistakes” (Cohen, 2000). Studies of medication errors have found that poor staffing, breakdowns in communication, inadequate staff education, and poor drug information contribute to the problem.
feared punishment for honestly reporting mistakes” (Cohen, 2000). Studies of medication errors have found that poor staffing, breakdowns in communication, inadequate staff education, and poor drug information contribute to the problem.
In response to President Clinton’s call for aggressive action to prevent medication errors, provisions have been made in the fiscal year 2001 budget. New spending in the amount of $16 million has been earmarked to address the problem. The Agency for Healthcare Research and Quality has been authorized to use $20 million in existing funds for research. These initiatives will focus on medical error prevention programs, patient safety research, reporting, and dissemination of information.
According to the Institute for Safe Medication Practices, a nonprofit organization that monitors and recommends safe medication practices, the following error-reduction strategies relating to chemotherapy administration have brought about positive changes in various institutions:
Only physicians who are experienced in the use of antineoplastic agents prescribe chemotherapy and manage the patient. In certain institutions where different levels of practitioners are allowed to write orders (oncology fellows, nurse practitioners), their orders are cosigned by the physician attending.
Chemotherapy is administered by professional staff members who are specially trained in chemotherapy administration. They should have completed an educational program according to their institutional policies and should comply with mandates for yearly competency requirements. In 1998, the Oncology Nursing Society developed a cancer chemotherapy course based on the society’s cancer chemotherapy guidelines and recommendations for practice. The course describes a didactic content and a clinical practicum needed to prepare the nurse to care for patients undergoing chemotherapy in various settings.
Orders are verified by at least two health care professionals, preferably one RN and one pharmacist. This involves a double check of the patient’s name, drug, dose calculation, route, frequency, total daily dose, and date of administration. Verbal orders for chemotherapy are not accepted.
The patient is identified positively by two health care professionals before drug administration. The patient’s identity
is verified by checking the identification band and the medical record number, and asking the patient to spell his or her name and give his or her address. In outpatient treatment centers, a photo attached to the patient’s profile card or an identification band can help identify the patient.
Patient education is a key component of the chemotherapy treatment. Patients are taught about side effects and self-care management and about the proper handling and disposal of chemotherapy agents.
Treatment parameters are identified and assessed before drug administration.
The use of a bar-code system has been shown to decrease medication errors. Computerized order entry systems are helpful; preprinted order sets are a good alternative.
Professional staff are oriented to the chemotherapy process and are instructed to follow standardized guidelines for writing chemotherapy orders, such as:
Use the drug’s generic name, not the brand name, acronym, or abbreviation.
Avoid decimals; round off to a whole number. If a decimal is required, use a zero before the decimal point (0.5 mg, not .5 mg).
Never use trailing zeros. If the dose is 20 mg, write it as “20 mg,” not “20.0 mg,” because the extra zero might be mistakenly added to the dose.
Refer to established maximum dose limits. If these are exceeded, specify the rationale and obtain necessary approvals as set by the institution.
Maintain good communication with the nurses and pharmacists who are involved in the chemotherapy administration system. If the order is “challenged,” the prescribing physician should not take it as an affront but as a helpful intervention to ensure the safety of the patient and the team.
Whenever new drugs or research protocols are started, diligent efforts are made to educate everyone involved about the protocol details and treatment regimen.
Hospital administrators should provide support for a multidisciplinary team of physicians, nurses, and pharmacists who are intimately involved in chemotherapy processes to work with a quality improvement professional. This team will analyze medication errors and recommend and promote institution-wide and system-based improvements to prevent errors.
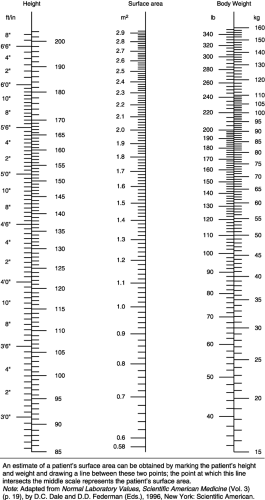
Most chemotherapy dosages are calculated based on body surface area (BSA) or weight (kg). The BSA, the external surface of the body, is expressed in square meters (m2). The BSA is more commonly used for dosing calculations because it is a more accurate measure of fluid and tissue proportion. To determine the BSA, a nomogram, a slide rule, or a BSA conversion calculator can be used. Throughout the institution, only one method should be used consistently to avoid variances in calculation, which might lead to dosing errors.
A nomogram (Fig. 4-1) is a table of three columns that correlates a person’s height and weight. The patient’s height is located in the left column, the weight is found in the right column, and a ruler is used to draw a line between the two. The point at which the line intersects the middle column represents the patient’s BSA. Because children differ in fluid and tissue proportions, a separate nomogram is used for children.
Chemotherapy doses are often ordered in milligrams per square meter (mg/m2). To calculate the dose a patient will receive, the drug ordered in mg/m2 is multiplied by the determined BSA:
Dose = (mg/m2) × m2
If a patient with a BSA of 1.60 m2 is ordered to receive paclitaxel 50 mg/m2, the patient would be given (50 mg/m2) × (1.60 m2) = 80 mg paclitaxel.
For obese patients, the ideal body weight (IBW) calculation may be used instead of the patient’s actual weight. The formula most often used is the following:
IBW (female) = 45.5 kg + (2.3 kg × [height – 60 in])
IBW (male) = 50 kg + (2.3 kg × [height – 60 in])
Dosing based on the area under the curve, used in ordering carboplatin doses, is explained in Unit II.
Before a chemotherapy treatment is initiated, the health professional responsible for the administration of the agent must independently assess the patient.
This pretreatment assessment and preparations must include the following:
The patient’s physical, cognitive, and emotional state
The patient’s knowledge of his or her disease and the treatment plan
The patient’s previous experience with chemotherapy or other treatment modalities
History of drug allergies
Presence of written informed consent if the patient is enrolled in a research protocol, or if required according to institutional policy. Informed consent verifies that the patient has been educated about the drug to be received and its benefits and risks.
Patient education needs, how these are going to be met, outcomes to be achieved, and plans for follow-up
Laboratory parameters and accepted ranges for drug administration (e.g., blood urea nitrogen and creatinine for patients who are to receive nephrotoxic agents) and the patient’s laboratory values before drug administration
A written physician’s order that includes the following:
Patient’s name and medical record number
Date the order was written and date of drug administration
Normalized chemotherapy dose calculated in mg/m2 or a unit of measurement, and the total daily dose to be given, not the total dose for the cycle
Route of administration
Frequency of administration
Length of time of administration
Medication orders for hydration, diuresis, anaphylaxis, emesis, and hypersensitivity reactions appropriate for the drug regimen
Readily available emergency equipment and medications should any adverse reactions, such as anaphylaxis, occur. A chemotherapy spill kit should also be within easy reach.
The patient’s name, date, drug, and dose on the label on the prepared agent and order verification by a pharmacist
Good venous access and verification of the intended use, patency, and integrity of the vascular access device, according to institutional policy.
After the pretreatment assessment is completed, the RN may administer the chemotherapy. As advances in chemotherapy occur, the routes of administration continue to evolve. The choice of drug route depends on the therapeutic intent. Peripheral and central lines are the most commonly used methods to administer chemotherapy. In the past 2 years, more oral cytotoxic agents have been discovered; oral administration makes it more convenient for patients to receive treatment at home and is less expensive but presents different challenges for the clinician. Alternative routes of drug delivery to specific sites in the body using devices or catheters are becoming more popular in clinical practice. This section discusses the methods of drug delivery, traditional and alternative routes, and practice implications for nurses.
ORAL
A wide variety of oral agents are now available. Capecitabine, a recent discovery, is an oral prodrug of 5-fluorouracil. A pro-drug is a chemical precursor of an active agent that needs to be converted into an active agent to become effective. The conversion is accomplished by one or more enzymes found in the liver, tumor sites, or other tissues. Prodrugs are believed to provide more selective and intensive therapy for the target tumor.
Most oral cytotoxic agents are self-administered by the patient at home, so the practice implications for the nurse involve patient education, monitoring for compliance, drug accounting, and documentation. Most of these functions involve intervention and are often accomplished by timely and skillful telephone triage.
INTRAMUSCULAR/SUBCUTANEOUS
Few chemotherapeutic agents are given using these routes. Some examples are leuprolide (subcutaneous), leucovorin (intramuscular), granulocyte colony-stimulating factor (subcutaneous), and several vaccines in immunotherapy. The nurse should monitor the platelet count because of the potential for bleeding, rotate injection sites to avoid further trauma, assess for infection, and ensure there is enough muscle
mass and tissue for absorption. Education is important so that the patient will follow these precautions.
mass and tissue for absorption. Education is important so that the patient will follow these precautions.
IV SIDE ARM
The IV side arm method involves the instillation of an agent into the side port (also referred to as the Y site) of a free-flowing IV line using a syringe. This is a common technique for vesicant administration. In the absence of blood return, administration must be stopped immediately to avoid injury through extravasation. If more than one agent is to be administered using this method, at least 10 mL of IV solution should be allowed to infuse between agents.
IV PUSH
IV push involves the direct administration of an agent into a central venous access device (CVAD) or peripherally into a vein using a butterfly needle or angiocatheter.
IV PIGGYBACK
The IV piggyback method involves the administration of an agent using a secondary set that is “piggybacked” into a primary line.
CONTINUOUS INTRAVENOUS INFUSION
Chemotherapy may be given by continuous infusion by means of peripheral access or a CVAD. Continuous infusions vary in length from hours to days, depending on the drug and protocol. The drug is usually in 250 to 1,000 mL of IV solution. To ensure a consistent flow and timely delivery of the drug, the infusion is usually hooked up to a gravity pump.
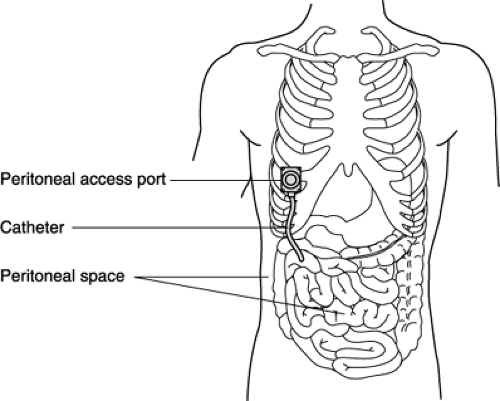
INTRAPERITONEAL CHEMOTHERAPY
Intraperitoneal (IP) chemotherapy is administered directly into the peritoneal cavity through a temporary catheter, an external intraperitoneal catheter, or an implanted port (Fig. 4-2). This route is useful for the treatment of intra-abdominal malignancies. There is a significant pharmacokinetic advantage in IP chemotherapy because it allows a lower peritoneal
clearance than plasma clearance of certain neoplastics, thus exposing the tumor to higher drug concentrations. There is also decreased potential for systemic toxicity because the cytotoxic drugs are metabolized in the liver before entering the systemic circulation.
clearance than plasma clearance of certain neoplastics, thus exposing the tumor to higher drug concentrations. There is also decreased potential for systemic toxicity because the cytotoxic drugs are metabolized in the liver before entering the systemic circulation.
Nursing Implications
Assess the patient for signs or symptoms of intra-abdominal infection: fever, abdominal pain, or discomfort. If these signs exist, do not administer the drug.
Assess the patency of the implanted port before administration. Noncoring needles (19G), usually 1″ long for average-size patients, are adequate to access the rubber diaphragm of the port. Longer needles are needed for obese patients.
Teach the patient about the side effects that may occur during and after drug administration (abdominal distention, pain, cramps, nausea, vomiting, diarrhea, constipation, shortness of breath, esophageal reflux). These symptoms will subside about 48 hours after completion
of treatment. The patient should also be instructed to turn from side to side every 30 minutes for 4 hours to promote drug distribution.
Maintain the patient in a semi-Fowler’s position.
Infuse the chemotherapy, which is added to 1 to 2 L normal saline at body temperature. Some patients report feeling cold during the infusion; blankets should be provided.
If severe abdominal pain occurs during the infusion of an agent that is either a vesicant or an investigational agent, stop the infusion and notify the physician.
Analgesics or antianxiety agents (e.g., meperidine or lorazepam) may be prescribed to relieve discomfort or anxiety during treatment.
Documentation should include date, time, drug, dosage, needle gauge and length, assessment of catheter patency, patient teaching and educational materials given, and patient’s tolerance of treatment.
INTRAHEPATIC CHEMOTHERAPY
This route of administration is indicated in patients with colorectal metastasis to the liver. Intrahepatic chemotherapy is administered in one of two ways: through a port catheter placed in the common hepatic artery, with the port body subcutaneously placed below the rib cage, or with a pump surgically implanted into a subcutaneous pocket in the abdominal wall (Almadrones et al., 1995). To administer chemotherapy through an arterial port catheter, a noncoring needle is used to access the device. To administer chemotherapy through a pump (by bolus or continuous infusion), a special noncoring pump needle is used percutaneously to access the pump’s flat, circular septum, which is connected to a drug reservoir. Hepatic pump models include the Medtronic, the Arrow, and the Infusaid pumps. By means of percutaneous injection, the pump reservoir is filled with an agent that is infused from the pump into a vein, an artery, or a body cavity (Fig. 4-3). The charging fluid in a small inner chamber is recharged intrinsically each time the pump is re-filled. When not being used to administer chemotherapy, this pump must be refilled every 2 weeks with an infusate solution to ensure that it remains charged and ready for use. The remaining infusate must be withdrawn from the pump
before more infusate can be administered and before the agent can be administered into the reservoir. The pump should be manipulated only by qualified personnel, who should follow the manufacturer’s instructions regarding proper use.
before more infusate can be administered and before the agent can be administered into the reservoir. The pump should be manipulated only by qualified personnel, who should follow the manufacturer’s instructions regarding proper use.
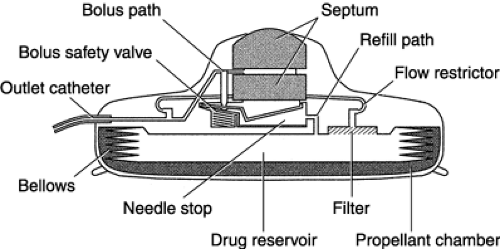 Fig. 4-3. Schematic drawing of the Arrow® implantable pump for intrahepatic chemotherapy administration. |
Flow rates range from 1 to 2 mL/day and are predetermined by the manufacturer. Flow rates increase at high altitudes, with fever, and with hypertension. Ambulatory hepatic infusion pumps can cause chemical hepatitis, chemical cholecystitis, and cholangitis.
Nursing Implications
Teach the patient how the pump works and precautions to take:
Avoid rough physical activity that might cause a blow to the pump site.
Do not place heating pads or hot-water bottles directly over the pump site. Avoid saunas or long baths. Increased temperature increases the pump’s flow rate.
Avoid deep-sea and scuba diving, because the increased pressure will affect the flow rate of the pump. Snorkeling and swimming are permitted.
Notify the physician or nurse when traveling by air, because the medication might need to be adjusted.
Emphasize the importance of keeping appointments for medication refills.
Before initiating treatment, a flow study must be undertaken and documented on the chart. The flow study verifies catheter placement and regional perfusion.
Access the pump septum with the special needle for the particular model being used. This is crucial to preserve the integrity of the self-sealing refill port.
Do not aspirate the pump. Aspiration causes blood to be drawn into the catheter and can result in catheter occlusion.
Record the drug residual at every visit. The residual volume is used to calculate the delivery rate. It also serves as a check for proper pump function.
Document the date, time, infusate, amount, patient’s response during and after the procedure, and patient teaching and educational materials given.
INTRATHECAL/INTRAVENTRICULAR
Drugs such as methotrexate, cytarabine, and thiotepa are commonly given by these methods. These routes are indicated in the management of patients who have leukemia and lymphomas of the central nervous system. An intrathecal catheter can be attached to an implanted port or pump. The Ommaya reservoir (Fig. 4-4) is an implanted ventricular device that allows the percutaneous injection of cytotoxic drugs directly into the lateral ventricles of the brain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree