Fig. 19.1
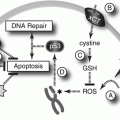
The Rho GTPase cycle of activation and inactivation. Rho GTPases in the inactive GDP-bound conformation can be converted to the active state by the GEF family of proteins. Rho GTPases in the active GTP-bound conformation can be converted to the inactive state by the RhoGAP family of proteins. RhoGAPS can act as inactivators or repressors of Rho GTPase function or as co-effectors
While many of the RhoGAPS are localized to or highly expressed in the central nervous system (CNS) (Moon and Zheng 2003), others are expressed ubiquitously. A few are most highly expressed in other organs, such as testis, spleen or liver. Many RhoGAPS are highly expressed in both brain and testes (Moon and Zheng 2003). All RhoGAPs contain a highly conserved ~150 amino acid RhoGAP catalytic domain. Many RhoGAPs are multi-domain containing proteins (Tcherkezian and Lamarche-Vane 2007) with one RhoGAP domain and one or more others such as START, StAR (steroidogenic acute regulatory)-related lipid transfer; IQ, calmodulin-binding motif; Lim, a zinc-binding domain; ral BR, a Ral GTPase-binding region; or S/T Kinase, serine/threonine kinase domain. This may facilitate convergence of multiple regulatory pathways by interaction with these motifs, thus promoting rigorous regulation of the GAP activity. While there are approximately 75 RhoGAP domain proteins that regulate the 20 known Rho GTPases, only a few have been extensively characterized (Boettner and Van Aelst 2002).
Preoptic Regulatory Factor-2 (Porf-2)
Discovery and Early Characterization
Porf-2 was originally discovered as part of a cDNA library made from the hypothalamus of the castrated male rat (Nowak 1990). A peptide of 8–9 kd was translated in vitro using an RNA that was transcribed in vitro from a 375 base cDNA template (Nowak 2003). This peptide was isolated and purified by centrifugal fractionation followed by high pressure liquid chromatography and characterized by capillary electrophoresis and polyacrylamide gel electrophoresis as an 8.3 kd peptide with an isoelectric point of 8.0. A 10.5 kd peptide was also expressed in bacteria using a pFLAG construct. A 16 kd native peptide detected with an anti-Porf-2 antibody was also identified in the preoptic-anterior and medial basal hypothalamus, hippocampus, cerebral cortex, cerebellum and testes of 70 day old rat (Nowak 2003).
Distribution of Porf-2 mRNA and Gene
A subsequent series of investigations yielded substantial information about the expression and regulation of Porf-2 mRNAs. Multiple transcripts ranging in size from 300 to 1,100 bases were detected, many of which were tissue specific. Although expression was highest in the brain regions of the hypothalamus and hippocampus, Porf-2 mRNAs were also found to have a wide tissue distribution, including, in the rat, other brain regions such as cerebral cortex and cerebellum, adrenal gland, testes, liver, and kidney, as well as human hypothalamus, adrenal, prostate, placenta and testis and cultured cells including cos-7 monkey kidney fibroblast-like cells, mouse cerebellar stem cells and Fischer rat thyroid-like (FRTL-5) cells. Table 19.1 summarizes the known sites of Porf-2 expression and the methods of detection used. Using gene dosage analysis, Porf-2 was shown in the rat to be a single copy gene (Nowak et al. 1997) Southern blot analysis indicates the presence of homologous DNA loci in rat, mouse, chicken, pig, sheep, cow, human and zebra fish (Schmerr et al. 2002). With the completion of the genome sequences for human and mouse, it was revealed that there are gene sequences in these two species that are highly homologous to rat Porf-2. These have been given various names, including KIAA_1688 in human and D15Wsu169e in the mouse (Peck et al. 2002). Table 19.2 lists the known phylogenetic distribution sites of Porf-2 and how these were determined. The retention of Porf-2 in diverse branches of vertebrate species is suggestive of a selective advantage and therefore an essential function for this gene and its product(s) (Silander and Ackermann 2009).
Table 19.1
Tissue and cell type distribution of Porf-2
Tissue and cell type | Location and species | Method of determination |
|---|---|---|
Brain: | Cerebral cortex (R) | N, NPA |
Hypothalamus (R, H) | N, NPA, ISH | |
Hippocampus (R) | N, NPA | |
Cerebellum (R) | N | |
Amygdala (R) | ISH | |
Endocrine organs: | Testes (R, H) | N, ISH |
Testes (M) | ESTa | |
Anterior pituitary (R) | N | |
Placenta (H, M) | N, ESTb | |
Adrenal (H) | N | |
Other organs: | Kidney (R) | PCR |
Prostate (low levels) (H) | N | |
Liver (low levels) (R) | N | |
Skin (M) | ESTa | |
Cells | FRTL-5 cells (R thyroid origin) | PCR |
Pancreatic beta cells (H) | PCRc | |
cos 7 cells (nhP kidney origin) | PAGE | |
C17.2 cells (M cerebellar stem cells) | PCR, WB | |
GT-1 cells (M hypothalamic neuronal origin) | N |
Table 19.2
Phylogenetic distribution of Porf-2
Group | Animal | Method of determination |
|---|---|---|
Primates | Human | Southern blot analysis |
Northern blot analysis | ||
Genome sequencinga | ||
Monkey | Protein electrophoresis | |
Ungulates | Pig, sheep, cow | Southern blot analysis |
Rodents | Mouse | Southern blot analysis |
PCR | ||
Western blot analysis | ||
EST | ||
Genome sequencingb | ||
Rat | cDNA sequence analysis | |
Southern blot analysis | ||
Northern blot analysis | ||
PCR | ||
Birds | Chicken | Southern blot analysis |
Fish | Zebrafish | Southern blot analysis |
Porf-2 Encodes a Putative RhoGAP Domain
There is a sequence-predicted Rho GAP domain at the carboxy-terminal end of the largest predicted Porf-2 open reading frame in mouse, rat and human. As mentioned earlier, highly homologous proteins may be translated from this open reading frame in human, mouse and rat. The mouse and human proteins also contain an actin binding domain. The human protein includes a RNA splicing regulator domain in the amino terminal portion. As noted earlier, many RhoGAPs are multi-domain containing proteins (Tcherkezian and Lamarche-Vane 2007) and at least one other RhoGAP, Myr5, has an actin filament binding site (Moon and Zheng 2003). As has been found for other RhoGAPs, the multiple protein-protein and protein-lipid interacting domains may determine the location of the Porf-2 RhoGAP within the cell. This also facilitates convergence of several regulatory pathways by interaction with multiple motifs, thus promoting rigorous regulation of the GAP activity.
Neural Expression of Porf-2 Is Modified by Pituitary and Gonadal Factors
Expression of Porf-2 in the hypothalamus is regulated by gonadal and pituitary factors. Both castration and hypophysectomy of 40–45 day old male rats increase levels of a brain-specific Porf-2 transcript in the preoptic area of the hypothalamus (POA), but not in the medial basal hypothalamus (MBH) or the cerebral cortex (Nowak 1997). This suggests that testicular secretagogues, either directly secreted by the testes or indirectly due to stimulation of the testes by pituitary factors, suppress Porf-2 in the POA in intact animals.
Porf-2 mRNA is also expressed in the female rat brain (Nowak et al. 1997). In situ hybridization analysis of female rat hypothalamus shows distinct strand specific labeling with a Porf-2 antisense 3H-RNA probe in discreet cells of the lateral POA (Nowak et al. 1997). In female rats ovariectomized at 44 days of age, treatment with 17β-estradiol (E2) for 8 days results in an increase in Porf-2 mRNA in the POA and in the hippocampus, as measured by nuclease protection assay. Treatment with progesterone (P4) increases Porf-2 mRNA in both the POA and the cerebral cortex. Combined treatment with E2/P4 decreases Porf-2 mRNA in the POA and cerebral cortex, but increases the mRNA in the hippocampus. No difference is observed in intrinsic levels of Porf-2 mRNA in either the MBH or POA during the estrous cycle (estrous compared with proestrous), while the same animals do exhibit higher levels of gonadotropin releasing hormone (GnRH) in the MBH and lower levels of GnRH in the POA during proestrus compared with estrus.
Expression of Porf-2 in Rat Testes
In addition to those found only in the CNS, many RhoGAPS are highly expressed in both brain and testes (Moon and Zheng 2003). Tissue specific Porf-2 transcripts are expressed in the testis (Nowak 1990). Porf-2 mRNA is highest at 15 days of age, then declines substantially at 30 and 60 days, when the levels are approximately 40% of the 15 day value (Nowak et al. 1997). There is an additional 40–50% decline between 2 months and a plateau at 6 and 12 months. There is a further 60–75% decrease at 24 months. The mRNA has been localized by in situ hybridization analysis in the 60 day rat testes to the immature germ cells including spermatogonia and primary spermatocytes.
Hypophysectomy of 42 day old male Sprague-Dawley rats results in significantly increased testicular expression 12 days later when compared to intact 54 day males. This suggests that loss of the testiculotrophic pituitary hormones, luteinizing hormone and follicle stimulating hormone, results in increased levels of pro-apoptotic and anti-proliferative Porf-2. In a separate study that looked at the effect of hypophysectomy on specific Porf-2 transcripts (Nowak 1997), the relative levels of certain testes-specific transcripts were altered, but the functional significance of these changes awaits the detailed characterization of these transcripts. Another RhoGAP protein, tGAP1, is highly and uniformly expressed in testicular germ cells and may play a role in a basic cellular function such as nuclear import/export (Modarressi et al. 2004). However, Porf-2 is not uniformly expressed in germ cells and thus may play a role in testicular germ cell apoptotic deletion of damaged cells (Sinha Hikim et al. 2003).
Effects of Aging on Neural Expression of Porf-2 mRNA
Nuclease protection assays were done using a riboprobe for Porf-2 mRNA that corresponds to 375 bases in the 3’ RhoGAP domain. In some regions of the adult male brain, levels of Porf-2 mRNA change with age (Hu and Nowak 1994), but not in others. Of the four brain regions examined, the MBH and POA, the hippocampus and the cerebral cortex, levels are highest in the hippocampus. In this region, Porf-2 mRNA levels are 40 μg/g total RNA at 2 months of age (young adult), increase significantly at 6 (mature adult) and 12 months (middle age) by over 100% to approximately 91 μg/g total RNA, then decline to levels seen in the young adult at 24 months (aged). In the POA, Porf-2 mRNA levels are highest at 2 months of age, at 19 μg/g total RNA, then decline steadily to approximately 6.5 μg/g total RNA in the aged 24 month animals. In the MBH and cerebral cortex there are no age-related changes observed. In the MBH, levels of Porf-2 mRNA are around 19 μg/g total RNA at 2 and 24 months of age, and slightly lower, averaging around 13.5 μg/g total RNA at 6 and 12 months but this difference is not statistically significant. Levels in the cortex are in the range of 20–23 μg/g total RNA at every age examined. The physiological significance of these changes in a pro-apoptotic factor in the brain with age remains to be established.
Sexually Dimorphic Expression of Porf-2 During Development
Expression of Porf-2 mRNA has also been examined during the perinatal and peri-pubertal stages of development. Porf-2 is expressed in the rat brain as early as embryonic day E18–19 (Nowak and Gore 1999). The levels of Porf-2 mRNA in the hypothalamus, relative to cyclophilin mRNA, are in the same range, (fg/pg), as those of two well-characterized neuromodulators, GnRH and neuropeptide Y (NPY). Both age and gender-related differences are observed in the levels of Porf-2 mRNA during the perinatal period. Porf-2 levels in the POA are highest at E18–19 in males (0.8 fg Porf-2 mRNA/pg cyclophilin mRNA), while the highest levels in females (0.65 fg Porf-2 mRNA/pg cyclophilin mRNA) are observed on the first day of life, P0. The gender differences on these 2 days are significant. Porf-2 mRNA levels are also higher in females (0.47 fg Porf-2 mRNA/pg cyclophilin mRNA) than in males (0.32 fg Porf-2 mRNA/pg cyclophilin mRNA) on postnatal day P15.
The levels of Porf-2 mRNA in the POA are also significantly higher on E18–19 than all other days examined, P0, P5, P10 and P15, while those on P0 are significantly higher than on P10 and P15. In the MBH, Porf-2 mRNA is most abundant on E18–19 and P0 (~6.5 fg Porf-2 mRNA/pg cyclophilin mRNA) and decreases significantly on P5, P10 and P15. By P15, Porf-2 mRNA measures 3.1 fg Porf-2 mRNA/pg cyclophilin mRNA, a greater than 50% decrease. In contrast, NPY mRNA is increasing during the perinatal period and GnRH mRNA shows no change. The observed gender and age differences in Porf-2 mRNA in both regions of the hypothalamus are statistically significant and may well be physiologically relevant, as the perinatal period is a critical time in the development and differentiation of the mammalian brain. Neuroendocrine sexual dimorphism begins during this period. There are differences in size and number of neurons. Neurogenesis, as well as apoptosis, contributes to the final outcome.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree