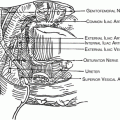
Nomenclature
Topography
Functional category
Treatment
Benign endometrial hyperplasia
Diffuse
Prolonged estrogen effect
Hormonal therapy, symptomatic
Endometrial intraepithelial neoplasia
Focal progressing to diffuse
Precancerous
Hormonal therapy or surgery
Endometrial adenocarcinoma, endometrioid type, well differentiated
Focal progressing to diffuse
Malignant
Surgery, stage based
Criteria | Comments |
|---|---|
Architecture | Area of glands greater than stroma (volume percentage stroma less than 55 %) |
Cytology | Cytology differs between architecturally crowded focus and background |
Size greater than 1 mm | Maximum linear dimension exceeds 1 mm |
Exclude mimics | Benign conditions with overlapping criteria (i.e., basalis, secretory, polyps, repair) |
Exclude cancer | Carcinoma if maze-like glands, solid areas, or appreciable cribriform |
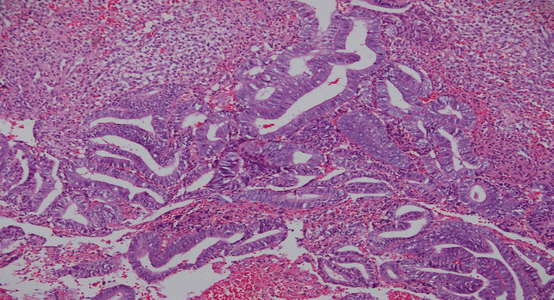
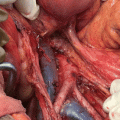
Fig. 3.1
Benign endometrial hyperplasia (Picture courtesy of Dr. Sandeep Mathur)
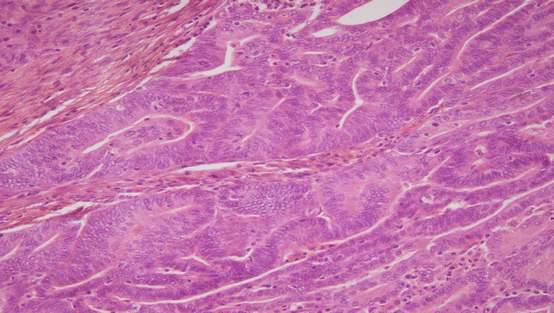
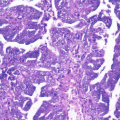
Fig. 3.2
Endometrial intraepithelial neoplasia (Picture courtesy of Dr. Sandeep Mathur)
Precancer Diagnosis: Endometrial Sampling and Imaging
The management of patients with premalignant endometrial lesions requires accurate diagnosis of a precancer lesion and exclusion of coexisting carcinoma to prevent any under- or overtreatment. Ideally, it should be possible to make this diagnosis preoperatively. However, it has been seen that in approximately 40 % of patients who had a diagnosis of endometrial intraepithelial neoplasia diagnosis by endometrial suction curette, the diagnosis changed to carcinoma after hysterectomy [8, 10], making exclusion of concurrent carcinoma a challenge.
Both dilatation and curettage (D&C) and endometrial suction curette have pitfalls in diagnosing precancer and excluding concurrent carcinoma. Both have sampling limitations: approximately 60 % of D&C specimens sample less than one half of the uterine cavity [11]. For women undergoing hysterectomy as a definitive management for premalignant lesions, the technique of sampling does not matter as much since hysterectomy eliminates the risk of failure to diagnose an endometrial cancer. Dilation and curettage and endometrial suction curette sampling devices have been reported to yield equal rates of cancer detection in patients with abnormal uterine bleeding [12]. The more accurate diagnosis of uterine lesions is made by hysteroscopy with directed biopsy as it helps in visual assessment of the background epithelium also [13–15]. It gives the best opportunity to confirm the diagnosis of a true premalignant endometrial lesion and exclude an associated endometrial carcinoma. Currently available diagnostic methods provide very little amount of endometrial tissue making cancer risk assessment less feasible. So it has been suggested that the assessment of sample adequacy should be included in the diagnostic scheme as is done for cervical cytology specimens.
In women with postmenopausal bleeding, transvaginal ultrasonography (TVS) is the most common employed imaging modality due to high specificity in excluding carcinoma. Endometrial sampling is not recommended if endometrial thickness is found to be 4 mm or less because of the very low risk of uterine malignancy in these patients [16]. An endometrial thickness greater than 4 mm in a patient with postmenopausal bleeding requires additional evaluation (such as sonohysterography, office hysteroscopy, or endometrial biopsy) to adequately visualize endometrial thickness. The significance of an endometrial thickness greater than 4 mm in an asymptomatic, postmenopausal patient has not been established, and this finding need not routinely trigger evaluation [16].
Unlike postmenopausal women, the role of TVS is limited in premenopausal women as endometrial thickness is not static during different phases of the menstrual cycle and may overlap with women having carcinoma.
The role of tumor markers for endometrial carcinoma is not well established. An inexpensive, sensitive, and specific serum test, which would be the most attractive approach to screen women for endometrial cancer, has still not been discovered. Raised serum CA 125 usually signifies an advanced disease and a poor prognosis but has limited role in monitoring treatment response. The serum markers CA 19-9, CA 15-3, and CA 72-4 and CEA levels are raised in endometrial cancer patients in 22–24 %, 24–32 %, 22–32 %, and 14–22 % of cases, respectively [17]. It has been seen that only a combination of CA 125 and CA 19-9 has a role in posttreatment surveillance due to high sensitivity (83.3 %) for detection of recurrence, with only 12.8 % of false-positive cases [17]. Tumor markers should be used in conjunction with other modalities, such as ultrasound and high-resolution MRI to attain high specificity.
Management of Endometrial Intraepithelial Neoplasia
Management of a newly diagnosed case of endometrial intraepithelial neoplasia has the following main objectives: (1) to exclude a concurrent adenocarcinoma, (2) to minimize the risk of delayed discovery of an occult carcinoma, and (3) to prevent progression to endometrial cancer.
Nonsurgical Management Options
Nonsurgical management is advised to patients (1) whose clinical, radiological, and pathological assessment suggests endometrial hyperplasia without any evidence of malignancy and (2) who desire future fertility (3) or patients with sufficient medical comorbidities precluding surgical management.
Presently nonsurgical management options include hormonal therapy and endometrial ablation. Endometrial ablation using thermal or electrical cautery devices has been employed for non-precancerous endometrial lesions, but it is not recommended for the treatment of atypical endometrial hyperplasia (AEH)/endometrial intraepithelial neoplasia (EIN). The completeness of ablation cannot be guaranteed via any method, and subsequent adhesions may make the cavity less accessible for follow-up surveillance.
Several studies have evaluated the use of hormonal treatment to induce regression of hyperplasia. Progestins are widely used with acceptable toxicity profile. Progesterone counteracts the mitogenic effects of estrogens and induces secretory differentiation [22]. Treatment with progestins may be an option for any patient who wants to retain childbearing, any patient with a hyperplastic or precancerous lesion who desires uterine preservation, and most elderly patients with medical comorbidities having diagnosis of endometrial intraepithelial neoplasia, a low-grade malignancy, or both. Although the efficacy of progesterone is well recognized, the exact dose and duration has not been specified till date [23–25]. Neither has the frequency been determined whether treatment should be cyclic or continuous. The appropriate length of follow-up after treatment also is still debatable.
Table 3.3 shows commonly used progestin regimes. Medroxyprogesterone acetate and megestrol acetate, with different doses and schedules, are the most common progestin therapies used in the clinical setting. Regression of hyperplasia (simple, complex, and atypical) has been observed in 80–90 % of individuals receiving medroxyprogesterone acetate (10 mg daily for 12–14 days per month) or micronized progesterone in vaginal cream (100 mg for 12–14 days per month) when treated for 3 months as shown in Table 3.3 [26–28]. Long-term systemic medical treatment to prevent reappearance of endometrial intraepithelial neoplasia requires awareness of concomitant adverse effects. Edema, gastrointestinal disturbances, and thromboembolic events are infrequent with these treatments, thereby making medical management a suitable therapeutic option for patients for whom surgical management is not desired. However, if endometrial intraepithelial neoplasia is present, there is a higher incidence of failure of medical management and subsequent development of cancer [29].
Table 3.3




Hormonal treatment for endometrial intraepithelial neoplasia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree