Citation
Number of DLGG (WHO grade II)
Clinical changes
Radiological changes
Increase in grade of malignancy
Daras et al.
3
Seizures (n = 1), Neurological deficit (n = 2)
Tumor recurrence (n = 3)
Yes (n = 3)
Isla et al.
2
Seizures (n = 2); Neurological deficit (n = 1)
Not detailed
Not detailed
Peeters et al.
32
Seizures (n = 17); Neurological deficit (n = 2); Increased intracranial pressure (n = 4); None (n = ?)
Imaging progression quantified on MRI follow-up (n = 28)
Not observed
Zwinkels et al.
9
Seizures (n = 3); Cranial nerve deficit (n = 1); None (n = 6)
Yes (n = 2); No (n = 7)
Not detailed
Yust-Katz et al.
14
Seizures (n = 7), Headache (n = 1), Neurological deficit (n = 1), None (n = 5)
Tumor progression (n = 4) out of the 8 cases diagnosed prior to pregnancy
Yes (n = 3) out of the 8 cases diagnosed prior to pregnancy
30.3 Effect of Pregnancy on a Glioma’s Natural History
The possible impact of pregnancy on a glioma’s behavior can be observed in clinical practice. It may result in clinical and morphological changes, possibly leading to additional oncological treatments.
Gliomas diagnosed during pregnancy are revealed by inaugural signs predominantly during the second or third trimesters of pregnancy [2–4, 18, 19], and patients with a glioma known prior to pregnancy may suffer from clinical deterioration during pregnancy [4, 13, 20]. However, the clinical presentation itself of gliomas during pregnancy appears comparable to the symptomatic picture outside the context of gravidity [1, 21, 22]. Indeed, seizures have been shown as a common presenting or worsening symptom for diffuse low-grade gliomas during pregnancy [3, 4, 10–12, 17, 21, 22] and have raised concerns regarding the risks of uncontrolled seizures and possible status epilepticus [4]. Beyond seizures, pregnancy has been reported to exacerbate neurological symptoms, which may precipitate an obstetrical emergency [4, 12, 15, 16]. Furthermore, concurrent eclampsia, although an extremely rare event, may initially mask the diagnosis of an underlying glioma presenting with seizures; it may also trigger the glioma to become symptomatic, through vasogenic cerebral edema, lowering the seizure threshold [16, 17].
In addition, pregnancy can induce morphological changes that are difficult to assess due to the lack of contrast-enhanced MR imaging during pregnancy. However, the quantitative analysis of the glioma growth over time can be performed easily by performing serial measurements of T2-weighted or FLAIR abnormalities over time, including during pregnancy, using a methodology previously described [23]. A previous study demonstrated in a subset of 12 WHO grade II gliomas that pregnancy increased the quantified imaging glioma growth rates in 75% of cases concomitantly with an increased seizure frequency in 40% of cases. The latter prompted further oncological treatment after delivery in 25% of cases [10]. Another study of 18 WHO grade II and III gliomas has shown that tumor progression on MRI follow-up, despite no quantitative measurement, occurs during or immediately after pregnancy in 44% of cases [11]. The change in tumor growth rates during pregnancy is illustrated in Fig. 30.1. Interestingly, a recent study noted an imaging progression, quantified as the imaging glioma growth rates, in more than 85% of total cases, with rates up to double as high during pregnancy compared to before pregnancy in more than 70% of total cases [14]. Hence, contrary to the dogma that pregnancy has not convincingly been shown to accelerate glioma growth [1, 2], the clinical deterioration and radiological tumor progression observed in this patient population, although transient in most cases, can prompt intrapartum oncological treatment. In this particular series 18% of patients with a glioma diagnosed during pregnancy were incited to get oncological treatment during their pregnancy, 70% of those patients received treatment shortly after delivery, and 25% of the patients with a glioma known prior to pregnancy [14]. Lastly, besides clinical deterioration and imaging progression, according to Daras et al., pregnancy may also lead to a transformation to a higher grade of malignancy in some of these patients [13]. Altogether, these findings clearly suggest a link between pregnancy and glioma progression, thus raising concerns for women with diffuse low-grade gliomas considering childbearing.
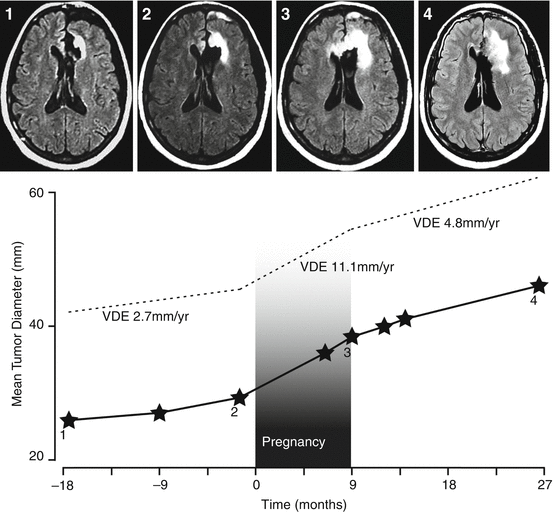

Fig. 30.1
Example of the evolution of glioma mean tumor diameter (mm) over time before, during (grey bar) and after pregnancy in a left frontal diffuse low-grade oligodendroglioma treated by subtotal surgical removal 27 months before pregnancy. Before pregnancy, the residual tumor grew continuously, with a velocity of diametric expansion of 2.7 mm/year. During pregnancy, the tumor growth rate increased, with a velocity of diametric expansion of 11.1 mm/year. After delivery, the velocity of diametric expansion decreased to 4.8 mm/year without adjuvant oncological treatment
30.4 Possible Mechanisms and Hormonal Factors Involved in Pregnancy-Related Effects on a Glioma’s Natural Evolution
The underlying mechanisms responsible for a glioma’s clinical and radiological changes observed during pregnancy remain unknown. One certainty, according to Ducray et al., is that the clinical worsening observed with pregnancy cannot only be explained by increased intracranial pressure since the latter tends to remain constant until delivery [1]. Three main hypotheses, possibly intermixed, can be suggested as responsible for the clinical and radiological worsening observed during pregnancy: immunological tolerance, steroid-mediated glioma growth, and hormone-mediated glioma growth [1, 13, 24, 25].
Possible interactions of pregnancy with gliomas have to do with the potential influence of sex hormones, which can cross the blood-brain barrier, and with physiological gestational changes [10, 13]. Specific steroid binding activity can be cancerous through activation of downstream expression of genes involved in cell growth, cell cycle progression and metastasis [13]. The levels of androgen, progesterone, and estrogen receptors seem to vary depending on glioma subtype and grade [13, 26, 27]. The presence of pregnancy-induced hormonal changes may lead to fluid retention, increased vascular volume, and peritumoral edema that may exacerbate an intracranial mass effect [1, 2, 10, 17, 28]. Some of the estrogen and progesterone receptors are cytosolic proteins [17]. Thus, they may mediate an increase in cellular dimensions through intracellular fluid retention, thereby contributing to tumor growth [17]. In addition, progesterone enhances glioma cell growth in humans [29] and expression of progesterone receptors increases with glioma grade as well as with invasion and migration markers [15, 27, 30]. Additionally, progesterone receptor positive gliomas have been shown to demonstrate high levels of Ki-67 expression [13, 27]. However, late manifestation of these gliomas, mainly during the second and third trimesters, would assume that the changes observed with pregnancy are likely related to more than just progesterone [17].
Contrarily, Cowppli-Bony et al. and Anic et al. found that low estrogen levels, whether earlier age at menarche, hormone replacement therapy, or oral contraceptive use, were consistently correlated with a decreased risk of tumor progression [25, 31]. The hypothesis behind these findings being that estrogen triggers growth inhibition and apoptosis in glioma cells through a multitude of mechanisms including cell cycle inhibition, decreased intracellular glutamate toxicity, and aromatase-mediated downregulation of astrocyte-derived TGF-beta1 and melatonin neuroprotection [25, 32, 33]. Furthermore, estrogen is also crucial to proper brain development during the gestational period, with a possible role in gliogenesis at that time [33]. Also noteworthy is that secretion of placental growth hormone at the maternal-placental interface stimulates secretion of growth factors, such as insulin-like growth factors, known to play a role in the proliferation and migration of glial cells, as well as secretion of pituitary gonadotrophins which also have receptors on tumor cells [4, 28]. Insulin-like growth factors 1 and 2 regulate and strongly stimulate glial cell migration and glioma growth [34, 35]. Peeters et al. found positive immunoexpression for insulin-like growth factor 1 receptor in 44% of pregnant patients with a diffuse low-grade glioma [14]. Though expression rate increased with WHO grade of malignancy, no clear correlation with glioma evolution during pregnancy, specifically clinical worsening and/or radiological progression, could be highlighted [14]. Of note, the latter study failed to find positive expression of estrogen, progesterone or growth hormone receptors in 20 diffuse low-grade gliomas of pregnant patients [14]. Results on the strict correlation of hormone receptors and tumor progression related to pregnancy remain inconclusive and no consensus has yet been reached.
30.5 Prognostic Factors and Survival Trends
A set of spontaneous prognostic factors in patients with diffuse low-grade gliomas has been isolated. The main estimated clinical, radiographic, or histopathological, predictors of both decreased survival without progression requiring oncological treatment, malignant-free survival and overall survival include male gender, older age (>40 years old), detrimental functional status (Karnofsky Performance Status <70), non-epileptic presentation (increased intracranial pressure, neurological deficit), absence of seizures at onset, non-frontal or parietal tumor location, large tumor size at diagnosis (>6 cm), tumor crossing the midline, rapid growth rates on MR follow-up (velocity of diametric expansion >8 mm/year), contrast enhancement (nodular-like or ring-like pattern), increased cerebral blood volume, presence of lactates and lipids on MR spectroscopy, astrocytic tumor subtype, high proliferative index, absence of 1p-19q codeletion, and absence of IDH1/2 mutation [36–43].
Interestingly, despite the previously discussed pregnancy-related effects on gliomas, pregnancy does not seem to markedly alter the outcomes of patients harboring a diffuse low-grade glioma. A recent retrospective cohort study on prospectively collected registry data identified 53 women who gave birth while harboring a diffuse low-grade glioma and showed that pregnancy does not seem to impact the overall survival in pregnant patients, compared to the control population of 247 women who did not give birth after the diffuse low-grade glioma diagnosis [44]. According to Peeters et al., no difference was found in progression-free survival between patients diagnosed with a WHO grade II glioma prior to pregnancy and those diagnosed during pregnancy [14]. Suggesting that early diagnosis does not give these patients a significant advantage with regards to time to tumor progression, and thus time of diagnosis and possible pre-pregnancy oncological treatment do not necessarily improve survivals. Therefore, whether a diffuse low-grade glioma was discovered prior to or during pregnancy should not impact the decision of when and how to treat the patient. Such outcomes could possibly be explained, or at least influenced, by the selection of female patients envisioning a pregnancy while harboring a glioma, including young age, good overall condition, tumor control at the time of pregnancy, and oncological treatments previously administered. In addition, the fact that female gender was isolated as a positive prognostic factor for diffuse low-grade gliomas cannot be ignored.
As previously mentioned, presence or absence of certain hormonal receptors and genetic markers can have some implications for patients’ prognosis. However, possibly due to a shorter follow-up, no associations were found between survival without progression requiring oncological treatment or overall survival and presence of histopathological markers and molecular alterations in diffuse low-grade gliomas during pregnancy [14]. Negative alpha-internexin and positive p53 immunoexpression were associated with at least double the radiological growth rates during pregnancy compared to the values before pregnancy [14]. In addition, along with high insulin growth factor 1 receptor expression, those markers tend to be associated with higher tumor grade [14]. Next, an increase of the radiological growth rates during gravidity correlated with prompted oncological treatments immediately postpartum. Thus, low grade gliomas with positive alpha-internexin, negative p53 expression, and low levels of insulin growth factor 1 receptor should be expected to have a smaller pregnancy-related increase in radiological growth rates, less likelihood of requiring oncological treatment promptly after delivery, and potentially a better overall prognosis.
30.6 Management Recommendations
Pregnancy makes oncological guidelines complicated as not all oncological treatments are approved for use in pregnant women. Despite controversy, different case series have reported pregnant women undergoing almost all combinations of oncological treatment types. In addition, management strategies are complicated by a variety of clinical and social factors [2, 14]. Consequently, these factors only allow for the assembly of an imperfect, practical, work-up plan for pregnant patients harboring a supratentorial diffuse low-grade glioma, using a multidisciplinary approach [1, 2, 4, 45]. An algorithm, adapted from Peeters et al., is proposed for management of the pregnancy in a patient diagnosed with a diffuse low-grade glioma prior to gravidity and during the pregnancy [14] (Fig. 30.2).