Introduction
One of the most common questions posed to thought leaders at continuous medical education meetings relates to the appropriate and timely management options for patients diagnosed with prediabetes. Patients frequently request information from their primary care physicians on ways that they may “cure diabetes.” Other patients proudly declare that they were once diagnosed with diabetes. However, with the use of lifestyle intervention, prescribed or herbal medications, they appear to be symptom and diagnosis free. Chiropractors are now advertising in local newspapers ways by which their homeopathic services may reverse diabetic peripheral neuropathy and type 2 diabetes (T2DM). With over 1.8 million Google hits for “prediabetes” versus only 434,000 for “prehypertension,” one could clearly understand the importance of early detection and prevention of diabetes. How one tests for, diagnoses, and treats prediabetes may have an enormous impact on a patient’s life quality and longevity.
Prediabetes is strongly correlated with increased insulin resistance, which is a mediator for an adverse cardiometabolic profile.
1 Prediabetes is characterized by decreasing sensitivity of target tissues to the action of insulin, elevated blood glucose, and insulin concentrations in association with elevations of hepatic glucose production and atherogenic lipids. Clinical markers of prediabetes include elevated plasma glucose concentrations under fasting and/or postprandial conditions, increased serum triglycerides, and below normal levels of high-density lipoprotein cholesterol (HDL-C). A decrease in antiatherogenic HDL-C is indicative of impaired reverse cholesterol transport that, over time, accelerates atherosclerosis.
Abnormal visceral adipose tissue secretions influence multiple metabolic pathways that modulate glycemia and blood pressure control. Ultimately, the anti-inflammatory and proinflammatory balance becomes disjointed favoring inflammation, impaired glucose tolerance (IGT), circadian abnormalities in blood pressure control, endothelial cell dysfunction, and abnormal platelet adhesion.
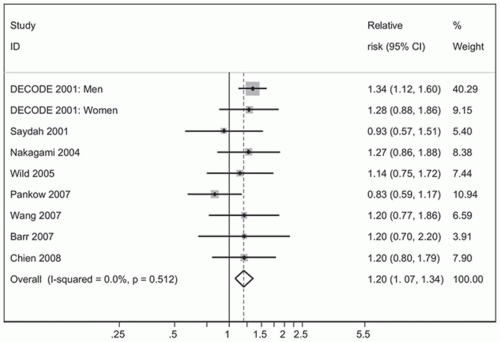
The estimated relative risk (RR) for cardiovascular disease associated with IGT ranges from 0.97 to 1.30 and that associated with IFG ranges from 1.12 to 1.37. The risk is higher for patients having fasting glucose levels in the range of 110 to 126 mg per dL (
Fig. 2-1).
2 Some reviews suggest that IGT doubles the risk for macrovascular disease.
3 The increased cardiovascular risk observed in patients with prediabetes is due to the endothelial changes that occur as one progresses toward clinical conversion to T2DM.
4Because patients with prediabetes convert to clinical diabetes at the rate of approximately 6% to 29% over 4 years, targeting such high-risk patients for early therapeutic interventions would be key to the primary prevention of coronary artery disease.
5Although one could argue about when and how to treat patients diagnosed with prediabetes, the truth of the matter is that in virtually all cases therapeutic interventions are safer, less expensive, and more durable when initiated earlier rather than later after one is diagnosed with IGT. As a physician, wouldn’t you rather treat an asymptomatic patient who presents for a routine physical exam and is found on screening to have a random blood glucose of 179 mg/dL and an A1C of 5.9%. The patient is overweight and has a family history of T2DM. Or, perhaps you would prefer evaluating a 65-year-old man, new to your practice with a 20-year history of poorly controlled diabetes and coronary artery disease?
Patients with prediabetes are often motivated to do whatever is necessary to avoid exposure to prolonged hyperglycemia and risking long-term complications. They know that early interventions, which normalize glucose levels will allow them to live lives comparable to those individuals who are euglycemic. Thus, these patients are eager to listen, learn, and adhere to any and all prescribed interventions including the adaptation of healthier lifestyles and pharmacologic therapies.

Prediabetes Definition and Prevalence
By definition, patients with prediabetes have evidence of impaired fasting glucose (IFG) (fasting glucose levels of 100 to 125 mg per dL), IGT [2-hour plasma glucose in a 75-g oral glucose
tolerance test (OGTT) of 140 to 199 mg per dL], or both IFG and IGT (
Table 2-1).
6 Normal fasting glucose levels are <100 mg per dL, while 2-hour postprandial glucose values do not exceed 140 mg per dL in euglycemic individuals. Any random blood glucose level >200 mg per dL is considered diagnostic for diabetes. Patients with prediabetes have a glycemic burden favoring the development of microvascular and macrovascular complications.
7,
8Six to ten percent of patients with IGT progress to clinical diabetes each year, whereas up to sixty-five percent of individuals with both IFG and IGT progress to clinical diabetes annually when compared to euglycemic subjects.
9 Therefore, aggressive screening and ambitious treatment of high-risk individuals targeting β-cell preservation may slow disease progression and theoretically improve outcomes.
Table 2-2 lists the risk factors for patients at risk for developing prediabetes as well as those individuals who warrant screening for the disorder. Progression rates of IFG or IGT to diabetes vary according to degrees of initial hyperglycemia, racial and ethnic backgrounds, and environmental influences. The higher the glucose values, the greater the risk of progression to diabetes and diabetic complications.
10Fifty-seven million Americans have prediabetes (IFG, IGT or both),
11 whereas the worldwide incidence of prediabetes is projected to be 418 million in 2025.
12As the prevalence of and progression to diabetes have increased, diabetes-related complications and disease state mortality have emerged as an expensive major health care issue. The annual cost of diabetes is over $174 billion. Direct costs related to diabetes and diabetes-related complications are $116 billion.
13 More importantly, diabetes robs individuals of productive days, months, and years that they could spend with family members and within the community as valuable contributors to society.
As primary care physicians, we are most adept at screening for, diagnosing, and managing chronic diseases. When physicians focus on identifying patients who are at high risk for developing diabetes and actually make the diagnosis of prediabetes, lives can be changed forever.
Ironically, the diagnosis of “prediabetes” may have some negative connotations. For example, once a patient is diagnosed as having IGT, his or her health insurance coverage may change because the patient now has a “preexisting condition” consistent with diabetes. Additionally, life insurance policies may become more expensive as underwriters adjust costs based on projected life expectancy rates that are influenced by a high prevalence of cardiovascular disease in patients with diabetes.
14Nevertheless, physicians should provide patients with a positive outlook after one is diagnosed with prediabetes. Patients with prediabetes have an opportunity to intensively manage their glucose intolerance at the earliest stages of the disorder while inducing either metabolic memory or a legacy effect. In the long run, patients will be less likely to develop the microvascular and macrovascular complications associated with prolonged exposure to hyperglycemia.
Despite the clear origins of diabetes-related complications early in the prediabetic state, no medications are approved by the U.S. Food and Drug Administration (FDA) for treating either IFG or IGT. Third-party payers may reject claims for lifestyle treatments or educational programs targeting diabetes prevention. Confusion exists among health-care providers as to the appropriate therapeutic and metabolic targets that should be achieved in patients with prediabetes. What is certain is that the risks of developing microvascular and macrovascular complications occur at levels of hyperglycemia much lower than those that are currently defined as indicative of clinical diabetes.

Screening for Prediabetes
Patients suspected of having prediabetes may be screened with a glucose level obtained following an 8-hour overnight fast or after a 75-g oral glucose challenge given in the morning after an 8-hour fast. Testing for IGT rather than IFG appears to increase the sensitivity of diagnosing prediabetes.
15In 2010, the American Diabetes Association (ADA) adapted the A1C as a screening tool for diabetes and prediabetes. Point-of-care A1C assays are not sufficiently accurate for diagnostic purposes. Therefore, testing should be performed using methods that are certified by the National Glycohemoglobin Standardization Program.
16 A1C assays are also more sensitive for diagnosing prediabetes than simply screening patients with fasting glucose. One study suggested that A1C screening of high-risk patients would successfully identify nearly 38 million Americans, initially labeled as having normal glucose tolerance (NGT) based upon fasting glucose values, as actually having prediabetes!
17A1C testing does have limitations as a screening tool. Compared with IFG and 2-hour postchallenge testing, A1C costs are approximately 50% higher. A1C levels can vary with patient’s ethnicity as well as with certain anemias and hemoglobinopathies.
18 Any condition affecting red blood cell turnover such as pregnancy, recent blood loss, transfusion, anemias, or sickle cell trait will cause A1C reporting to be inappropriately lower as shown in
Table 2-3.
Disease states such as renal insufficiency may directly affect A1C levels by interfering with the direct glycation of glucose to the hemoglobin structure. Alcohol ingestion may interfere with certain A1C assays, whereas elevated triglycerides artifactually decrease A1C levels.
19A1C has also been shown in prospective studies to predict progression toward clinical diabetes. In a systematic review following 44,000 individuals from 16 studies for up to 12 years, those with
A1C levels between 5.5% and 6% had a 5-year progression rate of 9% to 25%. A1Cs ranging from 6% to 6.5% at baseline progressed to clinical diabetes over 5 years at the rate of 25% to 50%, a rate 20 times higher than those having A1C levels <5.0%.
20 An A1C level >5.7% is associated with a risk of diabetes progression equal to that of the high-risk participants in the Diabetes Prevention Program (DPP). Thus, the ADA has defined any A1C in the range of 5.7% to 6.4% as having prediabetes, those most likely to progress clinically to diabetes. As with serum glucose measurements, cardiovascular risk increases in direct proportion to A1C. Interventions should be most intensive and follow-up particularly vigilant for patients with A1C levels >6%.
21Diagnostic tests for prediabetes and diabetes should be repeated to rule out laboratory error unless the diagnosis is unequivocal. A screened symptomatic patient with an A1C of 7.9% and a random blood glucose of 265 mg per dL can simply be treated for diabetes. However, a patient with a screening A1C of 6.2 % and a normal 2-hour 75 gram post-glucose challenge value below 140 mg/dL should be re-screened. Preferably, the same test should be repeated for confirmation. If the initial A1C is 6.4% with the secondary screen performed 2 months later being 6.3%, the diagnosis of prediabetes is confirmed. One can also make the diagnosis of prediabetes if two different tests such as the A1C and the 2-hour post-glucose challenge are above the diagnostic thresholds.
Laboratory testing for plasma glucose values is far from perfect. The College of American Pathologists surveyed 6,000 commercial labs by retesting glucose values previously run on 32 different types of instruments. Using a comparable standardized glucose concentration specimen, 12% of the commercial labs would have reported values with a potential for misclassifying the patient’s level of glucose tolerance.
22 Thus, a patient who is noted to have a fasting plasma glucose value of 122 mg per dL should always be retested to make certain the patient is not classified inappropriately as having prediabetes.
Additionally A1C may be useful as a screening tool for patients who present to the emergency department for management of an acute illness. An A1C > 5.7% performed in the acute care setting has a sensitivity of 54.8% and a specificity of 71.3% for detecting prediabetes. An A1C of 6% has a sensitivity of 76.9% and a specificity of 87.3% for diagnosing diabetes in the acute care setting.
23Individuals with an A1C of 5.7% to 6.4% have a risk of developing clinical diabetes equal to that of the high-risk population in the DPP.
24 In the United States, over 79 million adults are at risk
for progressing to T2DM based on elevated fasting glucose or A1C levels as shown in
Table 2-4. An A1C of 6% to 6.5% increases the likelihood of diabetes progression 20-fold compared with those having A1C values of 5%.
20 High-risk patients should be counseled on ways to reduce their glycemic and cardiovascular risks in a timely and intensive manner.
Various professional societies have published their customized guidelines for prediabetes as shown in
Table 2-5. Primary care physicians should familiarize themselves with suggestions put forth by at least one professional society. Patient counseling will be subsequently based on establishing an appropriate and timely diagnosis of prediabetes. One must also understand that the management of hyperglycemia intensifies as patients cross over the threshold into clinical diabetes.
• Risk Scores That Predict the Development of T2DM
One might argue that costly interventions and resources which may delay the onset of diabetes should be reserved for those patients who are at highest risk for disease progression. Detecting IFG identifies approximately 26% of the population as “at risk,” yet the annual rate of progression to diabetes within this subset of patients is only 5.56%.
25 The OGTT for detecting IGT is more specific than IFG as an indicator of diabetes risk but is difficult to perform in clinical practice. The use of fasting glucose, OGTT, and A1C in combination identifies over 80 million Americans as being at risk for developing diabetes.
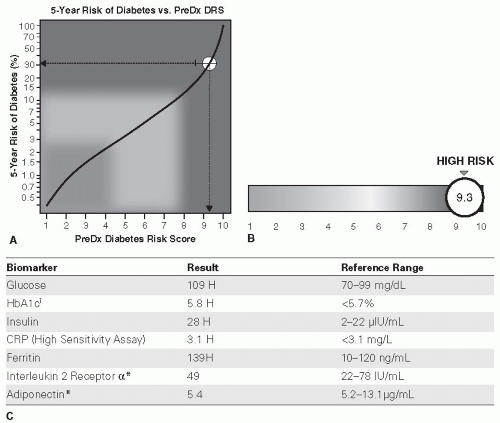
26The 2-page PreDx Diabetes Risk Score (DRS) (Tethys Bioscience, Inc., Emeryville, CA) is a multibiomarker blood test that may be used in patients with prediabetes to assess the 5-year risk of developing T2DM.
27 The following seven biomarkers of the PreDX DRS are quantified in a fasting blood sample:
Plasma glucose
Insulin
A1C
Adiponectin
Interleukin-2 receptor α
C-reactive protein
Ferritin
An algorithm, which incorporates results of the seven biomarkers as well as gender and age, is used to generate a DRS between 1 (least risk) and 10 (highest risk) that provides a quantitative measure of a patient’s likelihood of developing diabetes within 5 years. The algorithm was originally derived from a study that enrolled 6,784 persons in a 5-year diabetes outcomes study
28,
29 and was then verified in two additional cohort studies involving an additional 2,837 subjects.
30,
31The 2-page PreDx report (
Fig. 2-2) provides scores in terms of absolute risk (%) of progression to diabetes over 5 years, as well as one’s risk of progression relative to the general population. The results of the seven biomarkers used to calculate the DRS are also provided in the report. From
a clinical perspective, results of the test may allow appropriate tailoring of intervention programs, with the most intensive interventions implemented in the highest risk individuals.
Table 2-6 summarizes the utility of tests used to screen and diagnose prediabetes, diabetes, and one’s risk of progression to clinical diabetes.

Is Screening for Prediabetes Cost-effective?
If the benefit of preventing or delaying diabetes can be translated into long-term health benefits by preventing diabetes complications, screening and treating prediabetes will become a worthwhile public health initiative. The costs that will be borne by individuals and society as a result of missing the opportunity to diagnose prediabetes are unclear. However, the cost-effectiveness of early screening has been examined. A study by Zhang et al. suggested that the costs for screening for prediabetes or undiagnosed diabetes are <$200, with the cost per case being lower if only patients with higher body mass indexes (BMIs) were tested.
32 Patients who screen positive for prediabetes can be introduced to cost-effective interventions such as lifestyle modification, weight loss programs, and
metformin. These noninvasive therapies will result in a per quality-adjusted life-year gained savings for screened individuals of over $8,000.
33The DPP projections also show clear cost-effective benefits for both lifestyle and metformin interventions implemented to delay or prevent diabetes.
34 Clearly, the benefits of early screening and lifestyle interventions outweigh the risks in patients at high risk and should be considered part of a patient’s routine annual physical evaluation within the primary care setting.