Fig. 1
Sequence of the pre-analytical steps. From the surgical theaters, tissue specimens are transferred to Pathological Anatomy. Grossing is immediately followed by fixation and paraffin embedding. After sectioning of the tissue blocks, sections are appropriately stained. So far, only paraffin embedding and staining are standardized being run with dedicated apparatuses, while for the other steps ample variations of modalities, times, and protocols are used in different laboratories
Local variations in the practice of these steps are common in pathology laboratories worldwide and depend on type of tissue, traditional habits, requests by clinicians and patients and on specific investigations. Moreover, the goal of shortening the turn-around time (TAT) has to be considered.
In common practice, diagnostic and TAT requirements, hence processing, are different for “small” biopsies (<2 cm in size) and large, surgical resection specimens. A fast and reliable pre-surgical diagnosis is requested for the former and, accordingly, these biopsies are best collected directly in fixative-filled vials and soon transferred to the laboratory for paraffin embedding and routine diagnosis.
The processing of “large” surgical specimens is instead more critical, variable, and problematic, involving steps significantly affecting the preservation of antigens and nucleic acids.
This implies that fixation and processing of “small”, pre-surgical biopsies, which starts immediately after procurement, is relatively standardized. At variance, the handling of surgical specimens is more critical and undefined. This will be the topic of the present analysis.
The present interest on the analytical definition of the steps involved in the preparation of surgical specimens is linked to novel clinical requests, demanding genomic and antigenic testing for targeted molecular therapies. Indeed, requests for exploitation of archival formalin-fixed paraffin embedded (FFPE) tissues from surgical resections of breast, lung and colon cancers are exponentially increasing and Pathologists should get ready to play an important role in sample standardization in the years ahead. As a result, guidelines will have to be adopted by laboratories and hospitals on a global scale. Standardization of the pre-analytical phase of surgical specimens’ preparation would be the right answer.
2 The Ischemia Time
The time interval between surgical intervention and proper fixation of the removed specimen is defined as “ischemia time” and is crucial, since ischemia allows activation of tissue enzymes, autolysis, and degradation of proteins and nucleic acids [1, 2]. Substantial RNA degradation may occur during this time interval [3].
The “ischemia time” is currently subdivided into the “warm ischemia time” which occurs during operation, after ligation of blood supply till removal of the specimen from the body, and the “cold ischemia time,” afterwards. The former varies considerably (from a few minutes up to hours), depending on the complexity of the surgical procedure, ability of the surgeon, modality of intervention. The tissue remains alive and reactive, but will undergo progressive metabolic stress due to hypoxia. In an experimental study on colon cancer, Bray et al. [4] emphasized the need of protocols for tissue procurement and showed that significant variation in the level of expression (either increased or decreased) of transcripts was detectable already after 15 min, and by 120 min there was a fourfold increase in a number of genes with a more than twofold change in the level of expression. Indeed, this is bound to heavily influence the interpretation on the involvement of specific genes in the pathogenesis of diseases. For example, it is suggested that reduced expression of KLF6 may be implicated in the early stages of the molecular pathogenesis of approximately one-third of colorectal carcinoma cases, but the study by Bray et al. [4] shows that expression of KLF6 mRNA is significantly increased by delay in sample procurement.
The “cold ischemia time” is instead defined as the time interval between tissue removal from the patient and arrival in the pathology laboratory for grossing. During this interval it is mandatory to avoid autolysis and drying of the surface, which might damage tissue structure and components. The temperature of the specimen gradually reaches external temperature (either room temperature or 4 °C for tissues left in fridge), but this process is rather lengthy, as can be tested by using a needle thermometer (Fig. 2).
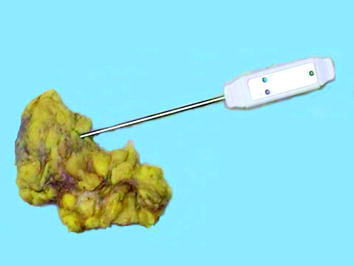

Fig. 2
Evaluation of the inner temperature in a surgical specimen, using a needle thermometer. Tissues (and notably fat tissue) are poor heath transmitters
Methods of transfer of surgical specimens vary according to the architectural layout and distance between surgical theaters and pathology laboratories. The ideal situation is when physical location and hospital protocols allow for the immediate transfer of “fresh” tissue specimens for prompt grossing and fixation. Accordingly, it has been recommended the “cold ischemia time” to remain below 1 h so as to allow a proper processing of breast cancer specimens and permit a correct evaluation of both morphological and therapeutic-prognostic parameters [5, 6]. Preservation of HER2 reactivity seems less critical than that of Estrogen (ER) and Progesterone Receptors (PgR), since optimal results are still attainable after a 3 h delay [7]. Problems arise when the “cold ischemia time” cannot be kept into properly defined limits: depending on the hospital structure the transport of surgical specimens from the surgical theater to the pathology lab may prolong the ischemia time. In addition, transfer is currently performed wordwide using the most variable types of boxes, transport media, and at different temperatures. It is common practice in many hospitals to immerse surgical resection specimens and organs into large formalin-filled containers, which are then transferred to the pathology laboratory in due time, usually once everyday. This practice carries problems, since:
1.
Plastic containers are large and relatively heavy; spilling may occur.
2.
Immersion of the whole specimen into formalin prevents the collection of fresh material for tissue banking. In addition, fixation does begin, but only at the periphery. A delay in the transfer to pathology is somehow justified by the fact that “the tissue is already in formalin”.
3.
Surgical nurses are becoming increasingly concerned about potential toxicity and carcinogenicity of formalin, since the fluid has to be handled outside the hood.
4.
When the container does arrive at the pathology lab, opening of the boxes, and handling of the specimen is major cause of diffusion of formaldehyde fumes.
Toxicity of formaldehyde is a matter of concern. This reagent demands caution since it is a skin allergen and produces irritating vapors that can cause asthma. Moreover, the International Agency for Cancer Research [8] has classified formaldehyde as a Class 1 carcinogenic agent. A positive relationship between formalin and respiratory symptoms has been reported not only in workers in match factories [9], but also in hospital staff members professionally exposed to this substance [10]. Statistical evidence has been presented for a possible link between formaldehyde exposure and lympho-hematopoietic malignancies [11], an observation that might fit with data reporting an excess of deaths due to cancer of the lymphatic and hematopoietic systems among British pathologists [12]. Still, the major concern for formaldehyde use is linked to the production of toxic, irritating, and allergenic vapors.
To ride over these problems, we adopted procedures designed to avoid the transfer of formalin-filled boxes in the hospital premises. We accordingly proposed to seal fresh surgical specimens under-vacuum in plastic bags in the surgical theaters, immediately after removal, and to keep them cooled at 4 °C until transfer to the pathology labs, where they are routinely processed [13]. Sealing of tissues in plastic bags is a quick procedure, taking approximately 15 s and is easily performed by nurses [14].
3 Under Vacuum Sealing
Under-vacuum sealing (UVS), per se, does not guarantee preservation, as experienced by Kristensen et al. [15]. Vacuum sealing, by removing air, prevents dehydration and favors cooling, the latter being the main preserving factor by blocking enzymatic autolysis. In fact, we experimentally demonstrated that cooling down at 4 °C is quicker in UVS-treated tissues [16]. Moreover, cooling down of under-vacuum sealed specimens can be speeded up by using a properly devised procedure (Fig. 3).
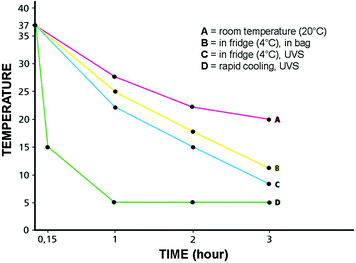

Fig. 3
Variations of the inner temperature in a surgical specimen (approximately 7 cm in diameter) in different conditions. From the body temperature, on surgical removal, if left at room temperature (A), it takes approximately 3 h for the specimen to stabilize at the external temperature. When the specimen is left, free, in a fridge (B), the inner temperature will gradually go down. The cooling process in Under-Vacuum Sealed (UVS) specimens (C) is facilitated by the lack of insulating air. By using cooling (-20°C) elements (D), the cooling of the UVS specimens is very rapid
Additional benefits are linked to the possibility of standardizing fixation times and of implementing tissue banking. In fact, we can now determine the starting time of fixation in formalin, thus avoiding over-fixation, which can affect immune-phenotyping of the specimen, an issue that is presently regarded as mandatory for breast cancer processing. A bonus of this under-vacuum Sealing and Cooling (UVSC) procedure is the preservation of RNA, which is permitted by the storage at 4 °C [17, 18], thus favoring tissue banking and gene expression profiling. Moreover, the UVSC procedure proved valuable for the preservation of hypoxia resistant cells, such as human stem/progenitor cells, which were successfully isolated and cultured from kidneys stored UVSC up to 48 h after surgery [19]. It derives that this procedure can be exploited to render the extraction of stem cells from human samples more practical and feasible. UVSC could also represent a reliable source for creation of primary cell cultures. The environmentally safe UVSC collection, preservation, and storage of surgical specimens may represent a strategy to bridge diagnostic and experimental pathology thus offering a new tool for bio-banking and for creation of clinically relevant models of neoplastic lesions (primary cultures) [16].
The UVSC procedure has extensively been adopted at the Azienda Ospedaliera Città della Salute e della Scienza di Torino (Italy), a large regional “pavilion” hospital where the distance between surgical theaters and pathology laboratories prevents an immediate transfer of fresh specimens. The transfer of surgical specimens across the Hospital in large formalin-filled boxes was a time-honored habit, but since 2009 the procedure of under-vacuum sealing and cooling has been adopted as the sole and routine procedure for the transfer of surgical specimens (larger than 2 cm). The experience accrued has been duly analyzed and reported [14]. The survey on the feasibility, compliance, and quality assurance of this new procedure for transferring surgical specimens was definitely positive. Dedicated apparatuses (TissueSafe®, Milestone srl, Sorisole, BG, Italy) were located in the premises of each of the six surgical theaters of the Hospital. The UVSC procedure was favorably accepted by the staff and did not present special problems of practical or diagnostic interest and was therefore adopted as the standard in the Hospital.
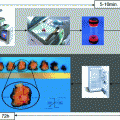
Moreover, the environmental goal of a progressive reduction of the exposure for nurses, pathologists, and technical personnel to formaldehyde vapors was met. The use of formalin has been reduced and restricted to dedicated areas in the pathology laboratory, and transfer of large boxes filled with fixative throughout the hospital ceased. The flowchart presented in Fig. 4 illustrates the procedures currently practiced in our Institution for tissue transfer and preservation.
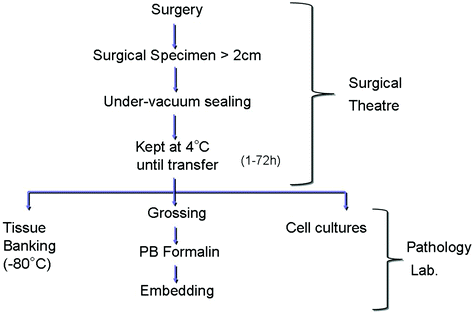

Fig. 4
Outline of the procedure for handling of the large (>2 cm) surgical specimens, as practiced in the Azienda Ospedaliera Città della Salute e della Scienza di Torino since year 2008. Under-Vacuum Sealed specimens are kept cool (in a fridge) in the surgical premises for a time variable from 1 up to 72 h (during weekends). Grossing, fixation, and histological processing follow. In alternative, material for tissue banking or culturing can be obtained from fresh tissues (PB phosphate buffered)
In conclusion, the simple UVSC processing offered advantages in terms of staff satisfaction, tissue preservation and cost.
4 Tissue Fixation
Fixation is the process whereby cell and tissue structures, as well as chemical components, are preserved in their integrity. This process is most commonly accomplished by immersion into a fluid, which gradually penetrates and acts chemically on the tissue components.
Several fixatives have been proposed, but those presently practiced are either of the aldehyde cross-linking category or alcohol-based, the latter producing coagulation by water subtraction. Alcohol-based fixatives have the advantage of lack of toxicity and of a good preservation of nucleic acids, but show a poor penetration and reportedly result in an unsatisfactory preservation of morphological details [20]. Moreover, in our [21] and in others’ [20, 22] experience, the performance of some immunohistochemical tests is impaired. The alcohol-based substitutes which have been proposed are generally non-cross-linking [21, 23–25] and allow a better preservation of RNA sequences. However, they are inferior as far as morphological (and immunohistochemical) preservation is concerned, and we have to conclude that substitution of formalin with alternative fixatives cannot be foreseen at present.
A 4 % formaldehyde solution in 0.1 M phosphate buffer pH 7.2 (phosphate buffered formalin, PBF) has been adopted as the fixative of choice in histopathology being (relatively) cheap, easy to use, and reliable (it does not over-fix). It guarantees, in appropriate conditions, an optimal morphological preservation. Still, the finality of formalin fixation has evolved over time. Originally, optimal morphological preservation was the sole requirement, but in more recent times, with the advent of immuno-histochemical typing, reliable antigenic preservation is also required [26, 27]. As a consequence, the protocols of formalin fixation have become stricter.
This issue is particularly relevant in onco-pathology for the evaluation of factors predicting responsiveness to therapeutic treatments, and thus, fixation in PBF of breast cancer tissue blocks for no less than 6 and no more than 48 h is now required in order to guarantee an optimal evaluation of ER, PgR, and HER2 expression by immunohistochemistry [28].
In more recent times, a crucial request in cancer pathology has been nucleic acid preservation for gene expression profiling, with the goal of generating new and reliable diagnostic and prognostic parameters [2, 29]. Indeed, evaluation of proliferation activity and clinical evolution prospects in breast cancer are already attainable with molecular tests, and evidence has been presented that evaluation of predictive biomarkers (ER, PgR, HER2) by RNA analysis is fully matching that obtained by immunohistochemistry [2, 29].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree