Clinicians commonly administer one or the other of the two hypomethylating agents currently approved in the United States—azacitidine or decitabine—to patients with aggressive forms of myelodysplastic syndromes (MDS). However, there continues to be uncertainty about the optimal choice of agent, the best initial dose and treatment schedule, the role of hypomethylating agents in patients with more indolent disease, the most appropriate management of treatment-associated adverse events, and the most desirable approach to maintain responses. The evidence base supporting clinical decisions around these questions varies widely in depth and quality. This article discusses practical considerations for clinicians who use hypomethylating agents to treat patients with MDS.
The U S Food and Drug Administration (FDA) approved azacitidine for treatment of patients with myelodysplastic syndromes (MDS) in May 2004, and the FDA subsequently granted regulatory approval to decitabine for MDS in May 2006. The European Medicines Agency (EMEA) formally approved azacitidine for treatment of MDS and acute myeloid leukemia (AML) in March 2009, and either azacitidine or decitabine is now available in most countries in the developed world. As a class of drugs, hypomethylating agents are now second only to erythropoiesis-stimulating agents in the frequency with which they are prescribed for patients with MDS outside of clinical trials.
Despite widespread clinical use of azacitidine and decitabine, several important practical questions remain with respect to these agents, including uncertainty about optimal patient and drug selection, dose and schedule for administration, and management of adverse events such as treatment-emergent cytopenias. Ideally, such questions would be addressed individually in well-designed clinical trials. However, the multiplicity of issues, the ready commercial availability of the drugs, and the great interest in trials of newer agents mean that it is unlikely future clinical trials will be initiated to resolve all of these areas of uncertainty. In addition, the relatively short duration of the remaining patent life and marketing exclusivity for both azacitidine and decitabine in the United States likely will limit the industry funding available for such postmarketing studies.
This article discusses the authors’ current approach treating patients with MDS using hypomethylating agents. This approach is based on both published data and the authors’ own clinical experience, and comments should be interpreted in light of the fact that lack of definitive study results does not obviate the need to make decisions in the clinic.
Background
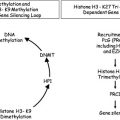
Azacitidine and decitabine are both potent inhibitors of DNA methyltransferases, a family of enzymes that have been highly conserved throughout evolution and are expressed in all tissues, and which are responsible for initiation and maintenance of 5′ methylation of cytosine nucleosides and associated gene silencing. Inhibition of DNA methyltransferases can result in hypomethylation of CpG dinucleotides in gene promoters and consequent reactivation of previously silenced genes. If some of these newly activated genes encode tumor suppressors or promoters of healthy cell differentiation, the consequences of reactivation of expression could be beneficial in MDS, since clonal hematopoiesis and impaired differentiation are central MDS-associated pathobiological features.
In addition to these epigenetic effects, both azacitidine and decitabine also have cytotoxic activity similar to the nucleoside analog cytarabine, as manifested by formation of γ-H2AX foci, a marker of double-stranded DNA breakage. The relative contribution of epigenetic mechanisms versus cytotoxicity to the clinical activity of the hypomethylating agents is currently unknown. A more detailed discussion of the biologic mechanisms of hypomethylating agents and their future prospects in neoplasia can be found (See the article by Jean-Pierre Issa elsewhere in this issue for further explanation of this topic.).
Clinical trial experience with either of the hypomethylating agents demonstrates complete responses in 9% to 37% of patients (using International Working Group 2000 criteria ), reduction in marrow blast burden without full hematological recovery in a similar proportion of patients, and hematological improvement in 20% to 48% of patients (overall objective response rates 30% to 73%) ( Table 1 ). In addition, about one third of patients with an abnormal karyotype experience complete cytogenetic remission during treatment with azacitidine or decitabine, indicating the potential for hypomethylating agent therapy to modify the natural history of MDS. Indeed, for patients with International Prognostic Scoring System (IPSS) intermediate-2 and high-risk MDS, the AZA-001 randomized trial demonstrated a 9-month overall benefit in median survival (ie, 24 months versus 15 months) for patients treated with azacitidine, compared with those who received supportive care alone. In contrast, a European randomized study with decitabine in higher-risk patients, GMDSSG/EORTC 06011, observed a modest 0.3-year improvement in median progression-free survival with decitabine treatment compared with supportive care, but failed to show improved overall survival.
| Trial | CALGB 9221 | AZA-001 | US Oncology | D-0007 | ICD03-180 (MD Anderson) | DACO-020 (ADOPT) | GMDSSG/EORTC 06011 |
|---|---|---|---|---|---|---|---|
| First author and year of primary publication | Silverman 2002, 2006 a | Fenaux 2009 | Lyons 2009 | Kantarjian 2006 | Kantarjian 2007 | Steensma 2009 | Wijermans 2008 |
| Number of patients enrolled | 191 | 358 | 151 | 170 | 95 | 99 | 233 |
| Number treated with hypomethylating agent | 150 b | 179 | 151 | 89 | 95 | 99 | 119 |
| Study type | Phase III (US registration) | Phase 3 | Phase 2 (randomized) | Phase 3 (US registration) | Phase 2 (randomized) | Phase 2 (singlearm) | Phase 3 |
| Drug regimen | Azacitidine SC 75 mg/m 2 daily × 7 d; total dose 525 mg/m 2 | Azacitidine SC 75 mg/m 2 daily × 7 d; total dose 525 mg/m 2 | Azacitidine SC over 5–10 d (3 arms) sparing weekends; total doses 375–525 mg/m 2 | Decitabine IV 15 mg/m 2 every 8 h × 9 doses; total dose 135 mg/m 2 | Decitabine IV or SC over 5 or 10 d (3 arms); total dose 100 mg/m 2 | Decitabine IV 20 mg/m 2 daily × 5 d; total dose 100 mg/m 2 | Decitabine IV 15 mg/m 2 every 8 h × 9 doses; total dose 135 mg/m 2 |
| Proportion with IPSS INT-2 or high-risk disease | 46% | 87% | IPSS not assessed | 70% | 66% | 46% | 93% |
| Proportion with de novo MDS | 80% | 100% | 100% | 87% | 70% | 89% | 88% |
| Median treatment cycles administered | >4 | 9 | 6 | 3 | 7 | 5 | 4 |
| CR rate (IWG 2000) in azacitidine- or decitabine- randomized group (where applicable) | 9% | 17% | NR | 9% | 37% | 15% | 13% |
| Overall improvement rate (CR + PR + HI) | 48% | 49% | >48% | 30% | 73% | 43% | 34% |
| Cytogenetic CR rate | NR | NR | NR | 35% | 35% | 33% | NR |
| Proportion of RBC transfusion dependent patients who became transfusion-free | 45% | 45% | 56% | 23% | NR | 33% | 32% |
a Ref. also includes data from earlier CALGB azacitidine trials (8421 and 8921).
b Ninety-nine patients were randomized to receive azacitidine; 51 additional patients initially randomized to supportive care later crossed over to receive azacitidine.
Current clinical trial efforts in MDS focus on improving response rates and response duration by combining hypomethylating agents with other biologically active agents, such as histone deacetylase inhibitors, for which there is in vitro evidence of synergy. A review of combination therapy is beyond the scope of the present discussion, but the initial results and prospects for histone deacetylase inhibitors in MDS are discussed in more detail in this issue (See the article by David P. Steensma elsewhere in this issue for further explanation of this topic.).
Which patients are most appropriate to treat with a hypomethylating agent?
In view of the demonstrated improvement in survival with azacitidine treatment of patients who have IPSS intermediate-2 or high risk MDS, individuals with higher-risk MDS are excellent candidates for hypomethylating agent therapy. Patients with 20% to 30% marrow blasts—currently classified as AML by the World Health Organization, but formerly classified as MDS subtype refractory anemia with excess blasts in transformation (RAEB-T)—also were included in the AZA-001 trial, and this group also experienced a survival benefit with azacitidine compared with supportive care.
It is not yet known whether a similar survival benefit will be accrued for lower-risk patients with MDS who receive azacitidine; nor is it known whether decitabine, when optimally administered, has an impact on survival comparable to azacitidine. When hypomethylating agent clinical trials have included lower-risk MDS patients, such as the CALGB 9221 azacitidine trial or the D-0007 decitabine registration study, the lower-risk patients have experienced similar overall hematopoietic response rates as were seen in higher-risk patients.
The lower-risk patients who have the most compelling indication for hypomethylating agent treatment are those patients who are transfusion-dependent, and for whom hematopoietic growth factors such as recombinant erythropoietin have failed. Reported red cell transfusion independence rates have ranged from 23% to 56% in transfusion-requiring patients treated with hypomethylating agents (see Table 1 ). Reduction of transfusion needs improves patient convenience, and also may delay or prevent the development of transfusion-related iron overload. There is considerable controversy at present, however, with respect to the actual clinical importance of iron overload in MDS relative to the other risks patients face, such as infection, bleeding, and disease progression.
Given the potential risks associated with hypomethylating agent treatment and relative paucity of active treatments in MDS generally, it seems prudent to withhold therapy from asymptomatic and minimally symptomatic patients with lower-risk disease, especially those who do not yet require transfusions. It is not yet known whether early treatment is better than late treatment in MDS. In hypomethylating agent trials enrolling patients with higher-risk disease, patients who were within 1 year of diagnosis had modestly higher response rates than those who had their disease for longer than 1 year, but it is not clear that a higher likelihood of response with earlier treatment also applies to lower-risk disease.
Another circumstance in which treatment with a hypomethylating agent may be appropriate is for patients with MDS who are candidates for allogeneic stem cell transplantation. Azacitidine or decitabine may stabilize disease for a few months while stem cell transplantation is coordinated and insurance approval is obtained, or, for patients who lack a sibling donor, while a search for an unrelated donor is ongoing. Because the median age of patients with MDS is 65 to 70 years, most patients with MDS who undergo stem cell transplantation receive reduced-intensity conditioning (RIC), in which the principle method of eliminating neoplastic cells is via a graft-versus-leukemia immunologic effect. Because patients who proceed to RIC stem cell transplant with less than 5% marrow blasts do better than those with higher marrow blast proportion, cytoreduction of patients with excess marrow blasts before conditioning also could represent an appropriate use of hypomethylating agents, although this hypothesis has not been tested formally. Although it also is not known whether it is beneficial to treat patients who already have less than 5% blasts before transplantation, the delays inherent in organizing an allogeneic transplant procedure make pretransplant hypomethylating therapy reasonable in this group, with the goal of temporary disease stabilization rather than cytoreduction.
Some physicians also use hypomethylating agents in patients without evidence of active disease—for example, to try to prevent relapse after induction chemotherapy in patients who experienced leukemic progression of MDS, or after stem cell transplantation. There are at present no data to support such an approach, and the authors feel that treatment of patients without active disease only should be considered in the context of a clinical trial.
Which patients are most appropriate to treat with a hypomethylating agent?
In view of the demonstrated improvement in survival with azacitidine treatment of patients who have IPSS intermediate-2 or high risk MDS, individuals with higher-risk MDS are excellent candidates for hypomethylating agent therapy. Patients with 20% to 30% marrow blasts—currently classified as AML by the World Health Organization, but formerly classified as MDS subtype refractory anemia with excess blasts in transformation (RAEB-T)—also were included in the AZA-001 trial, and this group also experienced a survival benefit with azacitidine compared with supportive care.
It is not yet known whether a similar survival benefit will be accrued for lower-risk patients with MDS who receive azacitidine; nor is it known whether decitabine, when optimally administered, has an impact on survival comparable to azacitidine. When hypomethylating agent clinical trials have included lower-risk MDS patients, such as the CALGB 9221 azacitidine trial or the D-0007 decitabine registration study, the lower-risk patients have experienced similar overall hematopoietic response rates as were seen in higher-risk patients.
The lower-risk patients who have the most compelling indication for hypomethylating agent treatment are those patients who are transfusion-dependent, and for whom hematopoietic growth factors such as recombinant erythropoietin have failed. Reported red cell transfusion independence rates have ranged from 23% to 56% in transfusion-requiring patients treated with hypomethylating agents (see Table 1 ). Reduction of transfusion needs improves patient convenience, and also may delay or prevent the development of transfusion-related iron overload. There is considerable controversy at present, however, with respect to the actual clinical importance of iron overload in MDS relative to the other risks patients face, such as infection, bleeding, and disease progression.
Given the potential risks associated with hypomethylating agent treatment and relative paucity of active treatments in MDS generally, it seems prudent to withhold therapy from asymptomatic and minimally symptomatic patients with lower-risk disease, especially those who do not yet require transfusions. It is not yet known whether early treatment is better than late treatment in MDS. In hypomethylating agent trials enrolling patients with higher-risk disease, patients who were within 1 year of diagnosis had modestly higher response rates than those who had their disease for longer than 1 year, but it is not clear that a higher likelihood of response with earlier treatment also applies to lower-risk disease.
Another circumstance in which treatment with a hypomethylating agent may be appropriate is for patients with MDS who are candidates for allogeneic stem cell transplantation. Azacitidine or decitabine may stabilize disease for a few months while stem cell transplantation is coordinated and insurance approval is obtained, or, for patients who lack a sibling donor, while a search for an unrelated donor is ongoing. Because the median age of patients with MDS is 65 to 70 years, most patients with MDS who undergo stem cell transplantation receive reduced-intensity conditioning (RIC), in which the principle method of eliminating neoplastic cells is via a graft-versus-leukemia immunologic effect. Because patients who proceed to RIC stem cell transplant with less than 5% marrow blasts do better than those with higher marrow blast proportion, cytoreduction of patients with excess marrow blasts before conditioning also could represent an appropriate use of hypomethylating agents, although this hypothesis has not been tested formally. Although it also is not known whether it is beneficial to treat patients who already have less than 5% blasts before transplantation, the delays inherent in organizing an allogeneic transplant procedure make pretransplant hypomethylating therapy reasonable in this group, with the goal of temporary disease stabilization rather than cytoreduction.
Some physicians also use hypomethylating agents in patients without evidence of active disease—for example, to try to prevent relapse after induction chemotherapy in patients who experienced leukemic progression of MDS, or after stem cell transplantation. There are at present no data to support such an approach, and the authors feel that treatment of patients without active disease only should be considered in the context of a clinical trial.
Once a decision has been made to administer a hypomethylating agent, which drug should be chosen?
In 2009, the National Comprehensive Cancer Network (NCCN) changed its MDS treatment guidelines ( http://www.nccn.org ) to recommend azacitidine as the preferred therapy for patients with higher-risk MDS, with decitabine an alternative. The NCCN made this change because of the survival advantage observed with azacitidine in the AZA-001 trial, and the unknown effect of decitabine on survival in MDS. The GMDSSG/EORTC 06011 decitabine survival study failed to show a survival benefit for reasons unrelated to the choice of hypomethylating agent. The reason for this may be because a decitabine regimen with suboptimal pharmacokinetics was used (ie, the 3-day inpatient regimen used in the D-0007 US registration trial and earlier phase 2 trials in Europe, instead of the widely used 5-day outpatient regimen developed at M.D. Anderson Cancer Center in Houston ). It is also possible that the trial was negative because many patients received a short duration of decitabine therapy (median four cycles of treatment in GMDSSG/EORTC 06011, with 40% of patients receiving two cycles or less, compared with a median of nine cycles in AZA-001), or because salvage chemotherapy was administered to more than 20% of patients who had been randomized to receive supportive care. Still, the trial’s negative result means that there are no studies demonstrating an overall survival benefit in MDS with decitabine treatment. The patent life and marketing exclusivity issues and other logistical considerations mentioned previously mean that it likely never will be known whether there is actually a survival benefit with decitabine in higher-risk MDS, so a preference for azacitidine seems appropriate, at least for patients who would have qualified for the AZA-001 trial.
Given the survival data with azacitidine, is there ever a reason to use decitabine? Leaving aside the fact that many clinicians have developed a comfort level with one agent or another because of local usage patterns, the highest complete response rate reported to date with a hypomethylating agent in MDS (37%, using IWG 2000 criteria) was observed in the single-institution MD Anderson study employing the 5-day 20 mg/m 2 /d intravenous decitabine regimen. This encouraging complete response rate continues to drive decitabine use. Idiosyncrasies of patient selection or trial design may be a factor in these results. A multicenter study of the same 5-day decitabine regimen showed a lower complete response rate (15%) compared with the single-center study, only slightly better than that reported with the 3-day decitabine regimen (9% to 13%).
It has been the authors’ impression that the intensity of therapy is greater with the commonly used 5-day decitabine regimen, compared with the 7-day 75 mg/m 2 /d azacitidine regimen (ie, these two outpatient regimens are not dose-equivalent). For instance, the rate of febrile neutropenia was higher in the ID03-0180 (14% of patients were hospitalized per treatment course, with 66% of patients hospitalized overall) and DACO-020 (alternative dosing for outpatient therapy [ADOPT]) (17% febrile neutropenia rate) 5-day decitabine trials, compared with CALGB 9221 or AZA-001 7-day azacitidine trials, in which there was no significant difference in the rate of febrile neutropenia between patients treated with azacitidine or those who received supportive care alone. In addition, the rate of response to decitabine seems to be somewhat more rapid: 82% of patients who would ultimately respond to decitabine in DACO-020 (ADOPT) had experienced an initial response by the end of two cycles, compared with 75% of responders showing improvement by cycle 4 of azacitidine in CALGB 9221, and 81% showing improvement after six cycles of azacitidine in AZA-001. Comparing across studies, however, is somewhat perilous, because enrolled populations may have differed. An azacitidine versus decitabine head-to-head trial is planned, which should answer some questions about relative pace of response and frequency of adverse effects, although the planned study will be too small to assess survival endpoints using noninferiority methods.
For the time being, the authors tend to prefer 7-day azacitidine in patients who would have been eligible for AZA-001 and for frailer patients, and 5-day decitabine in patients who appear to have more rapidly progressive disease. The authors discuss both agents with each patient, and consider patient preferences. They still use the older 3-day inpatient decitabine regimen studied in D-0007 and GMDSSG/EORTC 06011, but only rarely (eg, if the authors want to initiate a hypomethylating agent while the patient needs to be in the hospital for some other reason, or if patients prefer hospitalization to treatment in the outpatient clinic for logistical reasons).
If one hypomethylating agent has failed the patient, is there any reason to try the other? Despite the chemical similarity of azacitidine (5-azacitidine) and decitabine (5-aza-2′-deoxycitidine), which differ from one another only in a single hydroxyl group on the sugar moiety, there are biologic reasons why a patient might respond to one compound and not the other. Cellular metabolism of azacitidine and decitabine is similar, but not identical. Upon entry to the cell via cell surface equilibrative nucleoside transporters (ENTs), azacitidine is phosphorylated to 5-azacytidine monophosphate by uridine–cytidine kinase, whereas decitabine is phosphorylated by deoxycytidine kinase (the rate-limiting step in intracellular drug activation.) Low expression of deoxycytidine kinase correlates with decitabine resistance in neoplastic cell lines, but would not be expected to have an effect on azacitidine metabolism. In cell lines, sensitivity to decitabine correlates better with sensitivity to cytarabine than with sensitivity to azacitidine. In addition, the global pattern of hypomethylation induced in vitro by azacitidine is distinct from that induced by decitabine. These data suggest that there may be some patients who are destined to respond better to one hypomethylating agent than the other, and that some day it may be possible to obtain a gene expression signature before treatment to select the most appropriate agent. This approach, however, needs to be validated formally.
There is only one small published series describing the results of decitabine therapy for patients who were intolerant of, or had failed, azacitidine. In this report of 14 patients, 3 patients achieved complete remission, and 1 patient experienced hematological improvement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree