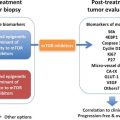
Agent
Phase
Clinical trial no.
Number of patients
Objective response rate (ORR, %)
Progression-free survival (PFS)
Overall survival (OS)
Reference
Temsirolimus
2
NCT00087074
41
5
2 months – median time to progression (TTP)
7.6 months
[54]
Cixutumumab + temsirolimus
2
NCT01016015
57 + 63
NA
A: 6.9 weeks
B: 10.6 weeks
C: 11.6 weeks median PFS
A: 18.9
B: 14.2
C: 14.7
months
[64]
Everolimus and imatinib
1–2
NCT00510354
Strata 1: 28
Strata 2: 47
NA
Strata 1: 17 %
Strata 2: 37 %
PFS at 4 months;
1.9 and 3.5 months median PFS
Strata 1: 14.9
Strata 2: 10.7 % median OS
[63]
Everolimus
2
NCT00767819
61
Arm 1: 13 %
Arm 2: 27 %
NA
NA
[60]
Ridaforolimus
2
NCT01010672
212
CBR –28.8 %
15.3 weeks – median PFS
40 weeks – median OS
[11]
Ridaforolimus
1/2a
NCT00112372
147 (85 sarcoma)
24.5 % – all pts.
27.1 % – sarcoma pts.
12.1 % – all pts.
17.1 % –sarcoma pts.
NA
[47]
Ridaforolimus
3
NCT00538239
711
1.3 % decrease in target lesion size vs. a 10.3 % increase with placebo
Ridaforolimus arm: 17.7 % vs. placebo arm: 14.6 weeks – median PFS
Ridaforolimus arm: 90.6 weeks vs. placebo arm: 85.3 weeks –median OS
[15]
Ridaforolimus has been the rapalog most extensively tested in sarcoma. Two phase 2 trials in patients with advanced sarcomas enrolling over 300 patients have reported six partial responses (two osteosarcoma, one spindle cell sarcoma, one malignant fibrous histiocytoma, one liposarcoma, and one follicular dendritic cell sarcoma) [11, 47]. The pivotal Sarcoma Multicenter Clinical Evaluation of the Efficacy of Ridaforolimus (SUCCEED) was designed to determine whether oral ridaforolimus can be used to maintain disease stability in the metastatic setting [15]. Among 711 patients enrolled, ridaforolimus treatment led to a statistically significant improvement in progression-free survival (PFS) compared with placebo (median PFS, 17.7 versus 14.6 weeks). Median overall survival (OS) with ridaforolimus was 90.6 weeks versus 85.3 weeks with placebo. Single-agent ridaforolimus was associated with a 29 % clinical benefit rate and 2 % partial response rate. Adverse events (AE) more common with ridaforolimus included stomatitis, infections, fatigue, thrombocytopenia, noninfectious pneumonitis, hyperglycemia, and rash. These toxicities are as expected for mTOR inhibitors.
In conclusion, mTOR inhibition in sarcoma patients may induce stable disease and, in a subset of patients, partial responses. The rarity of complete responses in patients indicates a cytostatic rather than cytotoxic effect for mTOR inhibition except in a small and as yet undefined subset of patients.
11.3 mTOR Inhibitors for the Treatment of Endometrial Carcinoma
Endometrial cancers are the most common gynecologic cancers in developed countries and third most common cause of gynecologic cancer death [48, 49]. Endometrial carcinomas are classified as type I and type II, based on clinical features and pathogenesis. Type I endometrial cancers occur most commonly in pre- and perimenopausal women often with a history of endometrial hyperplasia and exposure to elevated levels of estrogen. Type I endometrial carcinoma has an endometroid histology and is characterized by the presence of progesterone receptors and a benign biological behaviour. Type II endometrial carcinomas comprises types with high-grade serous and clear cell histologies, reduced/lack expression of progesterone receptors and originate from the mucosa, independently of hormonal stimulation [49]. Surgery is the primary treatment for resectable disease. Chemotherapy and radiation may be offered to women with high risk of recurrence following surgery. Chemotherapy and hormonal agents may be offered in the setting of recurrent/metastatic disease [48, 49].
Activation of the PI3K pathway occurs frequently in endometrial carcinoma through mutations in the catalytic and regulatory subunits of PI3K (PI3KCA, PI3KR1) and PTEN, suggesting an important role of these genes in the tumorigenesis [17]. Preclinical studies with ridaforolimus demonstrated antiproliferative activity in endometrial tumor cell lines [68]. In a mouse PTEN heterozygous model, everolimus significantly reduced endometrial hyperplasia and the proliferation index and significantly increased apoptosis compared with control [42].
Three rapalogs, everolimus, temsirolimus, and ridaforolimus, have been evaluated for activity in patients with recurrent/metastatic disease with/without prior chemotherapy (Table 11.2). In total, six phase 2 single-agent and one combination studies in patients with endometrial carcinoma have been reported. Among 44 patients with advanced endometrial cancer refractory to one or two chemotherapy regimens who received everolimus, there was a 36 % 3-month nonprogressive disease rate [59]. Four patients experienced partial responses. In a second trial, of 35 previously treated patients, the nonprogressive disease rate at 8 weeks was 43 %, and the median duration of nonprogressive disease was 4.5 months [66]. Median PFS was 2.8 months, and median OS was 8.1 months. The most common adverse events were anemia, fatigue, hypercholesterolemia, and lymphopenia. Thus, everolimus demonstrated some evidence of antitumor activity and acceptable tolerability in patients with chemotherapy-refractory advanced or metastatic endometrial cancer.
Table 11.2
Phase 2 trials in endometrial carcinoma
Agent | Phase | Clinical trial no. | Number of patients | Median duration of nonprogressive disease (months) | Median progression-free survival (PFS, months) | Median overall survival (OS, months) | Reference |
|---|---|---|---|---|---|---|---|
Everolimus | 2 | NCT00870337 | 44 | Response: 3.1 SD: 4.3 | 2.8 | 8.1 | [59] |
Everolimus | 2 | NCT00087685 | 35 | 4.5 | NA | NA | [66] |
Temsirolimus | 2 | NCT00072176 | Arm 1: 33 (chemotherapy-naïve disease) | Response: 5.1 SD: 9.7 | 7.33 | NA | [55] |
Arm 2: 27 (1 chemotherapy regimen) | Response: 4.9 SD: 3.8 | 3.25 | NA | ||||
Temsirolimus + hormone therapy | 2 | NCT00729586 | 20 (temsirolimus alone arm) | NA | NA | NA | [22] |
Ridaforolimus | 2 | NCT00122343 | 45 | Response: 29 SD: 4 | Na | NA | [12] |
Ridaforolimus | 2 | NCT00770185 | 35 | SD: 53 | NA | NA | [37] |
Ridaforolimus | 2 | NCT00739830 | 64 | NA | 5.6 | 9.6 | [56] |
Temsirolimus has been evaluated in two phase 2 trials. The first trial included patients who were chemotherapy naïve (group A) or who had received one prior line of chemotherapy for recurrent disease (group B) [55]. In the chemo-naïve group, four patients (14 %) had a confirmed partial response. In the chemotherapy-treated group, one patient had a confirmed partial response (4 %). Neither the loss of PTEN protein expression nor PTEN mutations evaluated from archival tumor specimens correlated with response. In the second trial, 3 of 21 previously treated patients had partial responses [22].
Ridaforolimus has been evaluated in two single-arm and one randomized phase 2 trials. In the first uncontrolled trial, there were two partial responses among 31 patients with endometrial carcinoma who had no prior chemotherapy [37]. In the second trial of 45 previously treated patients, 13 of 45 patients (29 %) had clinical benefit: 5 (11 %) with confirmed partial responses and 8 (18 %) with prolonged stable disease [12]. No correlation between PTEN protein expression and/or PIK3CA/AKT mutations and outcome was found. The interim report of the randomized phase 2 clinical trial comparing oral ridaforolimus with either hormonal therapy (n = 53) or chemotherapy (n = 13) [56] showed a median PFS of 3.6 months for patients receiving ridaforolimus compared to 1.9 months for those patients treated with hormonal therapy. No objective responses were reported for ridaforolimus. Ridaforolimus treatment was associated with higher toxicity rates, for hyperglycemia (19 %), fatigue, diarrhea, anemia, and mucositis. The results of these studies with ridaforolimus, everolimus, and temsirolimus suggest that mTOR inhibitors have consistent but modest single-agent clinical benefit in advanced and recurrent endometrial cancer.
11.4 mTOR Inhibitors in Gastric Cancers
Stomach cancer is the fourth most commonly diagnosed cancer and the second leading cause of cancer death worldwide [21]. Current management of localized gastric cancer is surgical resection with or without radiation and chemotherapy [40]. For patients with advanced unresectable disease and for patients that develop recurrent disease after surgery, chemotherapy may prolong survival and quality of life [2]. However, long-term outcomes of patients with advanced gastric cancer are poor, and thus, there is a need for novel targeted agents that may confer a better survival benefit.
Preclinical studies have shown dysregulation of mTOR activity in gastric cancer cell models and suggest that mTOR is a rational therapeutic target [3]. Mutations in upstream regulators of the mTOR signaling pathway, such as EGFR, amplification of human epidermal growth factor receptor 2 (HER2), PI3K, and PTEN, have been observed in patient-derived gastric tumor samples [13, 74]. Overexpression of the mTOR downstream effectors elf-4E and 4E-BP1 was shown in gastrointestinal cancer cells and primary tumors [16]. Others have shown that expression of phosphorylated mTOR protein in human gastric carcinomas correlated with tumor progression and poor survival [28, 34, 50]. Oncogenic transformation in tumors occurs with dysregulation of the mTOR pathway [8]. In addition, pharmacological inhibition of the PI3K pathway may induce an antitumor effect. Treatment of gastric cancer cell lines with the mTOR inhibitors sirolimus or everolimus was associated with an antiproliferative effect and decrease in phosphorylation of ribosomal protein S6 kinase 1 (S6K1) and 4E-BP1 and a reduction of HIF-1α and VEGF [10, 23, 39]. Everolimus treatment resulted in G1 cell cycle arrest and inhibited the proliferation of gastric cancer cell lines [35]. Consistent with the antiproliferative effects observed in vitro, mTOR inhibitors alone or in combination with other agents significantly delayed tumor progression in xenograft models of gastric cancer [10, 34].
Currently, everolimus is the only mTOR inhibitor that has been investigated in phase 1/2 clinical trials of patients with advanced gastric cancer (Table 11.3). In phase 1 trials, objective responses were seen with single-agent everolimus and in combination with mitomycin. Everolimus 10 mg/day resulted in a partial response with duration of more than 4 months in a heavily pretreated patient with gastric cancer and liver metastasis [53]. In a trial of everolimus (5–10 mg/day) plus mitomycin C, 3 of 13 evaluable patients (23 %) experienced a partial response, and 3 patients had stable disease [57].
Table 11.3
Phase II and III trials in gastric carcinoma
Agent | Phase | Clinical trial no. | Number of patients | Response rate or clinical benefit rate | Median progression-free survival (PFS, months) | Median overall survival (OS, months) | Reference |
|---|---|---|---|---|---|---|---|
Everolimus | 2 | NCT00519324 | 54 | 56 % (95 %CI) | 2.7 | 10.1 | [18] |
Everolimus | 2 | NCT00729482 | 54 | 18.4 (4-month PFS rate) | 1.7 | 8.3 | [76] |
Everolimus + BCS vs. placebo + BSC | 3 | NCT00879333 | 648 | 4.5 – everolimus arm 2.1 – placebo arm | 1.68 – everolimus arm 1.41 – placebo arm | 5.39 – everolimus arm 4.34 – placebo arm | [72] |
Paclitaxel + everolimus | 3 | NCT01248403 | 480 | NA | NA | NA | Clinicaltrial.org |
Two phase 2 single-agent studies have been reported in patients with advanced gastric cancer. In a recent phase 2 trial conducted in Japan, everolimus 10 mg/day was administrated to 53 patients with metastatic gastric cancer previously treated with one or two prior chemotherapy regimens [18]. Although no complete or partial responses were documented, 45 % of patients had a decrease in tumor size from baseline by independent radiologic review. Although median progression free survival was 2.7 months no complete or partial responses were obtained. At a median follow-up time of 9.6 months, median overall survival was 10.1 months. Everolimus monotherapy resulted in a promising disease control rate in patients with previously treated advanced gastric cancer [18].
A prospective, open-label, single-arm phase 2 trial (10 mg/day) evaluated the antitumor activity and the molecular determinants of responsiveness to everolimus 10 mg/day in heavily pretreated advanced gastric cancer patients (n = 54) [76]. Two patients (3.7 %) achieved partial response, and the disease control rate was 38.9 %. The high expression of pS6 (Ser240/Ser244) at baseline was significantly associated with higher disease control rate (DCR) and prolonged PFS [76].
Results from these phase 2 trials led to two randomized double-blind, multicenter phase 3 studies. In the first study (GRANITE-1, gastric antitumor trial with everolimus-1), patients with confirmed advanced gastric cancer and disease progression after one or two lines of systemic chemotherapy were randomized 2:1 to oral everolimus 10 mg/day plus best supportive care (BSC) or placebo plus BSC. The primary endpoint was OS. A total of 656 patients were enrolled, and 439 were randomized to everolimus and 217 to placebo. Median OS was 5.39 months with everolimus versus 4.34 months with placebo (HR 0.90; 95 % CI, 0.75–1.08, P = 0.1244). Median PFS per local investigator assessment was 1.68 months with everolimus versus 1.41 months with placebo. The response rates were 4.5 % with everolimus versus 2.1 % with placebo [72]. Everolimus monotherapy did not significantly improve OS in patients with advanced gastric cancer previously treated with one or two lines of systemic chemotherapy. The second phase 3 trial (RADPAC) is underway. It will evaluate paclitaxel monotherapy with or without everolimus in the second- or third-line setting [3]. The study has a target enrollment of 480 patients and the OS as the primary endpoint (NCT01248403).
11.5 mTOR Inhibitors in Bladder Cancer
Bladder cancer is the second most common malignancy of the genitourinary (GU) tract in men and is increasing in women [33] Greater than 90 % of bladder cancers diagnosed in western populations are transitional cell carcinomas of the urothelium (TCCU). TCCU is known to be sensitive to chemotherapy. The two first-line chemotherapy regimens for patients with locally advanced or metastatic urothelial carcinoma are a combination of gemcitabine and cisplatin (GC) or a four-drug combination of methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) [5]. In metastatic disease, chemotherapy is rarely curative and most patients with clinically localized cancers relapse after first-line therapy. The development of new therapies for treating patients with metastatic TCCU is a priority.
Aberrant activation of the PI3K-mTOR pathway may be involved in the progression of TCCU, as suggested by two recent studies [20, 70]. In one study, multivariate analysis showed that expression of pS6 and low PTEN expression correlated with shorter recurrence-free survival (RFS) in patients with high-risk non-muscle invasive TCCU [20]. Wu and colleagues reported that PTEN mutations are present in approximately 30 % of patients with TCCU and that the PI3K pathway regulated TCCU cell invasion [75]. In vitro and animal studies of everolimus and temsirolimus indicated antitumor activity in TCCU [38, 62]. These results suggest that the mTOR pathway is active in TCCU and provide a rationale for clinical trials targeting mTOR in this disease.
Clinical studies suggest that mTOR inhibitors have limited efficacy in unselected TCCU patients but may be active in a subset of patients with TCCU and tuberous sclerosis complex (TSC) mutations. Three studies of everolimus and temsirolimus have reported low response rates as single agents or in combination with chemotherapy in unselected patients (Table 11.4) [24, 51, 65]. Among 37 evaluable patients treated with single agent everolimus, one near-complete response, one partial response and several minor responses were seen and suggest that everolimus possesses biological activity in a subset of patients with bladder cancer. When whole-genome sequencing was used to investigate a complete and durable response in a patient with metastatic bladder cancer treated with everolimus, it showed a loss of function mutation in TSC1 (tuberous sclerosis complex 1), a regulator of mTOR pathway activation [31]. To maximize benefit from targeted agents such as everolimus, the preselection of patients based on molecular phenotype is required [43].
Table 11.4
Phase II trials in urothelial carcinomas
Agent | Phase | Clinical trial no. | Number of patients | Response rate or clinical benefit rate | Median progression-free survival (PFS, months) | Median overall survival (OS, months) | Reference |
|---|---|---|---|---|---|---|---|
Everolimus | 2 | NCT00805129 | 45 | 20 | 3.3 | 10.5 | [43] |
Everolimus | 2 | NCT00714025 | 37 | 5 | NA | NA | [65] |
Everolimus | 2 | NCT00933374 | 27 | 19 | 2.7 | 6.5 | [51] |
Temsirolimus | 2 | Eudra-CT 2008-008478-30 | 15 | NR | 2.5 | 3.5 | [24] |
11.6 Biomarker Studies in Clinical Trials with mTOR Inhibitors
On the basis of results from clinical trials, it is clear that the activity of mTOR inhibitors is limited to a subset of patients. As a result, there has been considerable research activity to identify markers that might predict sensitivity or resistance to mTOR inhibitors. To date there are no validated markers. Reasons for lack of successful identification of predictive biomarkers are multiple and include lack of correlation between preclinical models and patients and the likelihood that biomarkers of sensitivity and resistance to mTOR inhibitors are multifactorial and context specific. Recently reported preclinical and clinical studies in sarcoma, gastric, endometrial, and urothelial carcinoma have evaluated a number of potential candidate predictive markers (Table 11.5). These markers include genetic mutations and abnormal protein expression of various PI3KCA pathway components.
Table 11.5
Results of biomarkers studies from clinical trials
Marker evaluated – clinical studies | Results | |
|---|---|---|
Sarcoma | PD of ridaforolimus on p4EBP1 in surrogate normal tissue and tumor human specimens – phase 1 study [6] | Ridaforolimus induced a dose-dependent inhibition of p4EBP1 in PBMC, skin, and tumors that was associated with antitumor response |
p4EBP1 inhibition in PBMC [47] | No correlation between marker effect and antitumor activity | |
IHC of archival/fresh tumor samples for p27 Kip1, FKBP12, PTEN, pAKT, pS6, p4EBP1, pelF4E [11] VEGF levels pre-/post-dosing in blood samples | No correlation between archival tumor markers and CBR Blood VEGF levels show no correlation with CBR | |
pS6 levels in pre/post-temsirolimus treatment PBMC [54] | No significant relationship between pS6 and clinical outcomes | |
Endometrial | IHC protein expression for ER, PR, HER2, LKB1, PI3K, PTEN, pAKT, 4E-BP1, S6; FISH for PTEN [71]; DNA sequencing for KRAS, PIK3CA, PTEN, AKT1
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|