Introduction
Hyponatremia, defined as a serum sodium concentration ([Na + ]) less than 135 mEq/L, represents a relative excess of body water relative to body sodium content. The reported prevalence of hyponatremia varies with different patient populations and health care settings. Regardless, hyponatremia is still the most common electrolyte disorder seen in clinical practice and can significantly complicate a patient’s hospital course. The strong association between hyponatremia and in-hospital mortality has been demonstrated in numerous studies. Due to declining renal function, the presence of comorbidities, and high frequency of drug prescriptions, older patients are known to have a higher prevalence of hyponatremia. Data from the National Health and Nutrition Examination Survey (NHANES) also reveals that hyponatremia is more common in patients who have hypertension, diabetes, coronary artery disease, stroke, chronic obstructive pulmonary disease, cancer, and psychiatric disorders. Chronic hyponatremia, despite less severe symptoms than acute hyponatremia, is also associated with increased morbidity and mortality. A study involving more than 50,000 hospitalizations at a teaching academic medical center showed that even mild hyponatremia was associated with an increased in-hospital mortality, and that the risk of death was increased by 2.3% for each 1 mEq/L decline of serum [Na + ]. With an estimated annual cost ranging between $1.6 billion and $3.6 billion, hyponatremia has a substantial financial burden on the United States’ health care system. Among hospitalized patients, the presence of hyponatremia is associated with prolonged length of stay by 3.2 days and a 32% increased risk of hospital admission compared with patients without hyponatremia. Both the prolonged length of stay and increased hospitalization costs (including readmissions) account for approximately 70% of the total cost of the illness.
The central nervous system (CNS) plays a crucial role in the regulation of sodium and water homeostasis. The neurohypophysis consists of the vasopressin neurons in the hypothalamus that project via the pituitary stalk to the posterior pituitary, where arginine vasopressin (AVP) is secreted from the axon terminals. Disruption of neurohypophyseal regulation can result in conditions of salt and water imbalance, such as the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and diabetes insipidus (DI). These two phenomena have been well studied in the literature as potential risks after intracranial surgery; however, both can also occur after traumatic brain injury (TBI) as well.
Elderly patients and those with comorbidities are more at risk for developing electrolyte derangements, particularly hyponatremia. Hyponatremia predisposes patients to gait instability, which can lead to more frequent falls and increased fracture rates. , A study showed that a 5-mmol/L decrease in serum [Na + ] had about the same effect on falls as aging 13 years. Increased fracture rates are also due to chronic hyponatremia-induced deterioration of bone mass and strength, and increased fragility. Evidence has shown that even patients with mild hyponatremia (serum [Na + ] = 130 to 134 mEq/L) have an increased prevalence of osteoporosis. Epidemiologic analysis of the NHANES III database suggested that osteoporosis occurred at a significantly increased 2.5-fold odds ratio (OR) in hyponatremic subjects over age 50 compared with participants with a normal serum [Na + ]. A more recent epidemiologic study of 2.9 million electronic health records indicated that chronic hyponatremia was significantly associated with both osteoporosis and bone fractures at OR of 3.97 and 4.61, respectively. As chronic hyponatremia may not cause overt symptoms, it frequently remains undiagnosed and untreated until complications manifest. These complications can compromise postoperative rehabilitation, particularly in elderly patients.
Postoperative surgical patients and patients with TBI are also especially prone to the development of hyponatremia. Hyponatremia in the postoperative period is associated with worse surgical outcomes, including increased mortality. Hyponatremia has traditionally been managed by internists, intensivists, endocrinologists, nephrologists, and geriatricians. However, with the increased incidence of preoperative, postoperative, and posttraumatic hyponatremia and its known adverse consequences, timely recognition and management by other providers such as surgeons is crucial for optimal management of their patients. This chapter will specifically focus on the recognition, evaluation, and treatment of patients with postoperative and posttraumatic hyponatremia.
Definition and Classification of Hyponatremia
As a brief overview, hyponatremia is defined as a serum [Na + ] less than 135 mEq/L. Hyponatremia can be categorized by symptoms, serum [Na + ], and duration. The most useful categorization from the point of view of treatment is by symptoms: mild, moderate, and severe. Mild hyponatremia is serum [Na + ] less than 135 mEq/L with neurologic manifestations that can include headache, irritability, difficulty concentrating, altered mood, and depression. Usually mild hyponatremias are chronic (several days to many weeks/months), but these symptoms are also seen with early stages of more acute hyponatremias. Moderate hyponatremia includes symptoms of nausea, confusion, disorientation, altered mental status, and unstable gait with or without falls. Usually, the serum [Na + ] is less than 130 mEq/L with a duration of more than 48 hours. Lastly, severe hyponatremia has more advanced neurologic symptoms, including vomiting, seizures, obtundation, respiratory distress, and coma. This usually occurs with acute hyponatremia of less than 24- to 48-hour duration with serum [Na + ] less than 125 mEq/L ( Table 18.1 ). Of note, children are at a higher risk of developing symptomatic hyponatremia because of their larger brain-to-skull size ratio.
| Serum Sodium | Neurologic Symptoms | Typical Duration of Hyponatremia | |
|---|---|---|---|
| Severe | <125 mmol/L | Vomiting; seizures; obtundation; respiratory distress; coma | Acute (<24–48 h) |
| Moderate | <130 mmol/L | Nausea; confusion; disorientation; altered mental status; unstable gait/falls | Intermediate or chronic (>24–48 h) |
| Mild | <135 mmol/L | Headache; irritability; difficulty concentrating; altered mood; depression | Chronic (several days or to many weeks/months |
The etiology of underlying hyponatremia is usually classified based on three different components: the patient’s plasma tonicity, extracellular fluid (ECF) volume status, and the severity of hyponatremia in terms of the serum [Na + ] level. Plasma tonicity is further delineated into hypotonic, isotonic, or hypertonic, which is characterized by the relationship of the plasma osmolality to the serum [Na + ]. As hypotonic hyponatremia causes a shift of water from the ECF into cells due to osmotic gradients, only patients with hypotonic hyponatremia should further be differentiated based on ECF volume status (hypovolemic, euvolemic, and hypervolemic). Hypovolemic hyponatremia can occur in patients who have had gastrointestinal, renal, or fluid losses, diuretic therapy, and renal salt wasting. Euvolemic hyponatremia can be due to SIADH, hypothyroidism, exercise-associated hyponatremia, low solute intake, polydipsia, or the use of nonsteroidal antiinflammatory drugs (NSAIDS). Adrenal insufficiency can cause hyponatremia as a result of multiple factors, including renal salt wasting in primary adrenal insufficiency and impaired water excretion in secondary adrenal insufficiency. As a result, primary adrenal insufficiency causes hypovolemic hyponatremia, whereas secondary adrenal insufficiency causes euvolemic hyponatremia. Lastly, hypervolemic hyponatremia can occur in volume overload states such as congestive heart failure, cirrhosis, renal failure, and nephrotic syndrome. In these conditions, there is increased activity of AVP via baroreceptor-mediated nonosmotic stimulation caused by reduced effective circulating arterial volume.
Arginine Vasopressin
Arginine vasopressin (AVP), also known as antidiuretic hormone (ADH), is an essential peptide hormone that is synthesized in the hypothalamus and stored in the posterior pituitary gland, from which it is released into the circulation. AVP plays a major role in the regulation of water and sodium homeostasis by virtue of its antidiuretic action in the kidney. AVP secretion occurs either due to increased plasma osmolality or decreased arterial blood pressure and/or blood volume. Osmoreceptors are sensory receptors in the anterior hypothalamus that detect changes in osmotic pressure and contribute to maintaining fluid balance in the body. Changes that deviate by as little as ∼3 mOsm/kg from the set point (280 to 285 mOsm/kg H 2 O) trigger specific homeostatic responses to restore water balance. Baroreceptors, on the other hand, are mechanoreceptor sensory receptors that detect changes in blood pressure. A decrease in blood pressure greater than 10% to 15% is detected by baroreceptors in the cardiac atria, aorta, and carotid sinus, leading to a decrease in tonic inhibition of AVP release, which ultimately leads to AVP release from the posterior pituitary gland. AVP binding to AVP V2 receptors on the kidney collecting duct principal cells activates a signal transduction cascade that inserts aquaporin-2 (AQP2) water channels into the apical membrane of the collecting ducts, leading to reabsorption of water back into the circulation. This results in what is known as antidiuresis: decreased renal free water clearance, increased urinary concentration, and reduced urinary volume. Along with osmoreceptor-stimulated thirst, this process promotes normalization of plasma osmolality. The function of AVP is especially important during periods of severe blood loss, such as hemorrhagic shock, or systemic infection, such as sepsis, when there is a lack of responsiveness to other vasoconstrictors (which is where AVP gets its name vasopressin ). By binding to the V1a receptors, which are expressed on vascular smooth muscle, AVP also produces vasoconstriction and increased peripheral vascular resistance, thus promoting increases in blood pressure.
Either a deficiency or excess of AVP can result in clinical disease. Conditions such as SIADH result in secretion of AVP that is inappropriate for the plasma osmolality, which results in water retention, hyponatremia, and oliguria. SIADH is suspected when the diagnostic evaluation reveals euvolemia on clinical examination, plasma osmolality less than 275 mOsm/kg H 2 O, inappropriate urinary concentration (urine osmolality greater than 100 mOsm/kg H 2 O), and elevated urinary [Na + ] (greater than 30 mmol/L). SIADH is a diagnosis of exclusion, after other causes of euvolemic hypoosmolality (hypothyroidism and hypocortisolism) have been ruled out. SIADH is largely a clinical diagnosis that is supported by biochemical parameters. SIADH can be caused by underlying medical conditions such as CNS disturbances (e.g., stroke, hemorrhage, infection, trauma, and psychosis), malignancies (small cell carcinoma of the lung, head and neck cancer, olfactory neuroblastoma, and extrapulmonary small cell carcinomas), drugs (selective serotonin reuptake inhibitors, NSAIDs, certain anticonvulsants, certain chemotherapy agents), pulmonary disease (pneumonia, tuberculosis), and HIV infection.
In contrast, DI occurs either from lack of pituitary production of AVP (central DI) or renal unresponsiveness to AVP (nephrogenic DI). In either case, DI ultimately results in hypotonic polyuria (urine osmolality less than 250 mmol/kg) and hypernatremia. Central DI usually occurs due to traumatic, inflammatory, infectious, or cancer-related lesions that affect the neurohypophysis, whereas nephrogenic DI can either be inherited (e.g., mutations in the AVP V2 receptor gene or mutations in the AQP2 water channel gene) or acquired from hypokalemia, postobstructive polyuria, and medications such as lithium. DI results in polyuria with dilute urine and polydipsia.
Circulating concentrations of AVP under normal physiologic conditions range from less than 0.5 to 6 ng/L. Although AVP is present in both the blood and urine, its quantification for diagnostic purposes can be difficult. Copeptin is a peptide derived from the C-terminus of the AVP prohormone that is more stable in plasma. Given that is it secreted in equimolar amounts as AVP from the posterior pituitary and can be measured more easily in plasma, copeptin has emerged as a promising surrogate marker for AVP in the diagnosis of AVP-dependent fluid disorders. Copeptin measurements allow accurate differentiation between various conditions within the polyuria–polydipsia syndrome. In the absence of prior fluid deprivation, baseline copeptin levels greater than 20 pmol/L reliably identifies patients with nephrogenic diabetes insipidus.
General Treatment Considerations for Hyponatremia
Assessing the severity of hyponatremia based on a patient’s symptoms, rather than the serum [Na + ] concentration alone, is critical prior to determining the initial therapy for hyponatremia. Both the magnitude and rate of decrease in the serum [Na + ] concentration, as well as the chronicity of the hyponatremia, are correlated with the symptoms that a patient experiences. As discussed previously, symptoms related to acute hyponatremia are more severe than the symptoms of chronic hyponatremia. Altered mental status, seizures, focal neurologic deficits, coma, or other signs or symptoms of cerebral edema all meet criteria for hyponatremic encephalopathy (HNE). HNE is a medical emergency and should be treated urgently in order to prevent morbidity and mortality. Brain herniation from hyponatremia is seen almost exclusively in patients with acute hyponatremia or in patients with coexisting intracranial pathology. An increase in serum [Na + ] of 4 to 6 mEq/L is usually sufficient to reduce symptoms of acute hyponatremia. Chronic hyponatremia, if not complicated by an acute episode, rarely presents as severely symptomatic HNE. This reflects the brain adaptation during chronic hyponatremia with a negligible brain volume increase.
Acute hyponatremia with symptoms should be treated urgently with hypertonic (3%) saline, either via bolus or continuous infusion, in order to appropriately raise the serum [Na + ] and prevent catastrophic consequences such as cerebral edema, irreversible neurologic damage, respiratory arrest, brainstem herniation, and death. Currently, vasopressin antagonists, such as vaptans, have no role in the treatment of severely symptomatic hyponatremia because of the uncertainty of increasing the serum [Na + ] sufficiently rapidly to alleviate the neurologic symptoms. Additionally, vasopressin antagonists should not be given in hyponatremia due to hypovolemic conditions, such as gastrointestinal or renal losses, as they can exacerbate hypotension and hypovolemia.
Rapid correction of chronic severe hyponatremia may lead to osmotic demyelination syndrome (ODS; previously called central pontine myelinolysis) and even death. The clinical course of ODS is biphasic, beginning with an initial phase of encephalopathy or seizures, which is caused by the hyponatremia. These symptoms improve with correction of the serum [Na + ]; however, a few days later, there is significant neurologic deterioration due to the osmotic myelinolysis. This second phase manifests as motor impairments leading to quadriparesis and pseudobulbar palsy in the most extreme form. It is usually only partially reversible, and leads to high morbidity and potential mortality, thus prevention is extremely important. ODS can be confirmed with magnetic resonance imaging (MRI), as it can take the classic appearance of a hypointense pons on sagittal imaging but a hyperintense pons on coronal imaging. However, these changes may take 1 to 2 weeks to visualize by MRI.
In order to prevent the serious consequences of inadvertent overcorrection of serum [Na + ] in patients with acute hyponatremia, current guidelines recommend raising the serum [Na + ] concentration only by 6 to 8 mEq/L in the first 24-hour period. In symptomatic patients with acute hyponatremia or patients with severe symptoms, this goal should be achieved quickly (over 6 hours or less) with a constant serum [Na + ] level maintained for the remainder of the 24-hour period to avoid overly rapid correction. Most cases of ODS have occurred in patients with severe hyponatremia whose serum [Na + ] concentration was raised by more than 10 to 12 mEq/L within 24 hours or more than 18 mEq/L within 48 hours. An important exception are patients with coexisting risk factors for ODS (serum [Na + ] less than 105 mEq/L, hypokalemia, alcoholism, malnutrition, or liver failure), who should be corrected no more than 8 mEq/L in any 24-hour period. , In cases of rapid serum [Na + ] overcorrection, hypotonic fluids such as 5% dextrose in water can be given to match the rate of urinary output in order to slow the rate of correction. In cases where the urine output is profound, this can be supplemented by administration of desmopressin.
Hyponatremia in the Surgical and TBI Setting: Preoperative Hyponatremia
To date, most existing studies have focused primarily on hyponatremia in patients admitted to internal medicine services. The association between preoperative hyponatremia and perioperative outcomes in surgical patients remains less well known. However, one large-scale observational study evaluated the 30-day perioperative morbidity and mortality in patients who had documented preoperative hyponatremia. Out of 964,263 adult patients undergoing major surgery from more than 200 hospitals, 75,423 patients (7.8%) had preoperative hyponatremia (defined as a serum [Na + ] less than 135 mEq/L). The greatest prevalence of preoperative hyponatremia was seen in patients undergoing cardiac surgery (11.8%) and vascular surgery (11.2%), followed by general (7.5%), orthopedic (7.1%), and other (6.1%) surgical procedures. This study found that preoperative hyponatremia was associated with a higher risk of 30-day mortality, especially in patients undergoing nonemergency surgery. Furthermore, hyponatremia was associated with a greater risk of perioperative major coronary events, wound infections, and pneumonia. Lastly, perioperative hyponatremia prolonged median lengths of stay by approximately 1 day. This illustrates the importance of careful evaluation of patients’ electrolytes prior to surgery, and that even mild abnormalities in serum [Na + ] can have significant clinical consequences. Preoperative hyponatremia should be evaluated and treated prior to nonemergency surgical procedures if a reversible or treatable cause of the hyponatremia is found.
Hyponatremia in the Surgical and TBI Setting: Postoperative Hyponatremia
Postoperative patients represent another subset of patients who are vulnerable to the development of hyponatremia and its adverse consequences. Current knowledge of postoperative hyponatremia is based on small numbers of patients; therefore, more information is needed regarding the incidence, clinical setting, and outcome in larger surgical populations. Postoperative hyponatremia has previously been reported with prevalences of 1% to 5% in the United States and the European Union. In another study, out of 1088 operative procedures performed (including cardiovascular, gastrointestinal-biliary tract, renal transplantation, and neurosurgical), there were 48 episodes (4.4% prevalence) of postoperative hyponatremia. Specifically, patients who underwent organ transplants, cardiovascular procedures, and surgery for trauma or gastroenterological conditions were at a high risk of developing hyponatremia. Other studies have also shown a high frequency of postoperative hyponatremia in patients who have undergone spinal fusion surgery (20%), subtotal gastrectomy (67%), chronic biliary tube drainage (22%), mitral valve surgery (30%), and general surgery-trauma (40%). ,
The development of hyponatremia after any type of surgical intervention is due to multiple factors. These factors include the stress response to the surgical procedure itself, the loss of blood and other bodily fluids, and the administration of intravenous fluids and blood products during preoperative, intraoperative, and postoperative periods. Potent nonosmotic stimuli to AVP secretion, such as positive pressure ventilation, stress, nausea and vomiting, hypoglycemia, fever, or decrease in intravascular volume, are common after surgery and can additionally increase the risk of hyponatremia.
Numerous hormonal changes occur in response to surgery that influence salt and water metabolism during the postoperative time period. AVP release promotes water retention, and depending on the severity of the surgery and the development of complications, increased AVP secretion can continue for 3 to 5 days postoperatively. Additionally, renin is released from the juxtaglomerular cells of the kidney, partially due to increased sympathetic efferent activation. Renin stimulates the adrenal cortex to release aldosterone, which then leads to sodium reabsorption from the distal convoluted tubules in the kidney, with secondary water resorption.
Aside from elevated plasma AVP levels, the most common precipitant of hyponatremia in patients after surgery is the iatrogenic infusion of hypotonic fluids (such as 0.45% sodium chloride [NaCl] or 5% dextrose in water). This is due to the dilutional effect on the serum [Na + ] levels that occurs with hypotonic fluid administration in the presence of nonosmotic AVP hormone release. Even administration of isotonic solutions (e.g., 0.9% NaCl) can result in a paradoxical fall in sodium concentration as the sodium contained in these solutions is excreted in the urine. This results in the net retention of electrolyte-free water and, subsequently, hyponatremia, a process that has been termed “desalination.”
Neurosurgical Patients
Hyponatremia is the most common electrolyte disorder encountered in neurosurgical patients. It has been reported to occur in 50% of subarachnoid hemorrhage and 10% of transsphenoidal hypophysectomy cases. The acute onset of most neurosurgical illnesses (e.g., subarachnoid hemorrhage and TBI) implies that the hyponatremia is acute, and therefore these patients are more likely to be symptomatic. Patients’ symptoms can be further exacerbated by other factors that produce cerebral irritation, including increased intracranial pressure or neurosurgical interventions. Hypovolemic volume status in neurosurgical patients can be clinically assessed by central venous pressure (CVP) or the presence of hypotension and/or tachycardia. Hypervolemic volume status usually shows elevated CVP with signs of fluid overload, such as peripheral and/or pulmonary edema and a positive fluid balance. However, most neurosurgical patients will be euvolemic with SIADH as the major etiology of their hyponatremia.
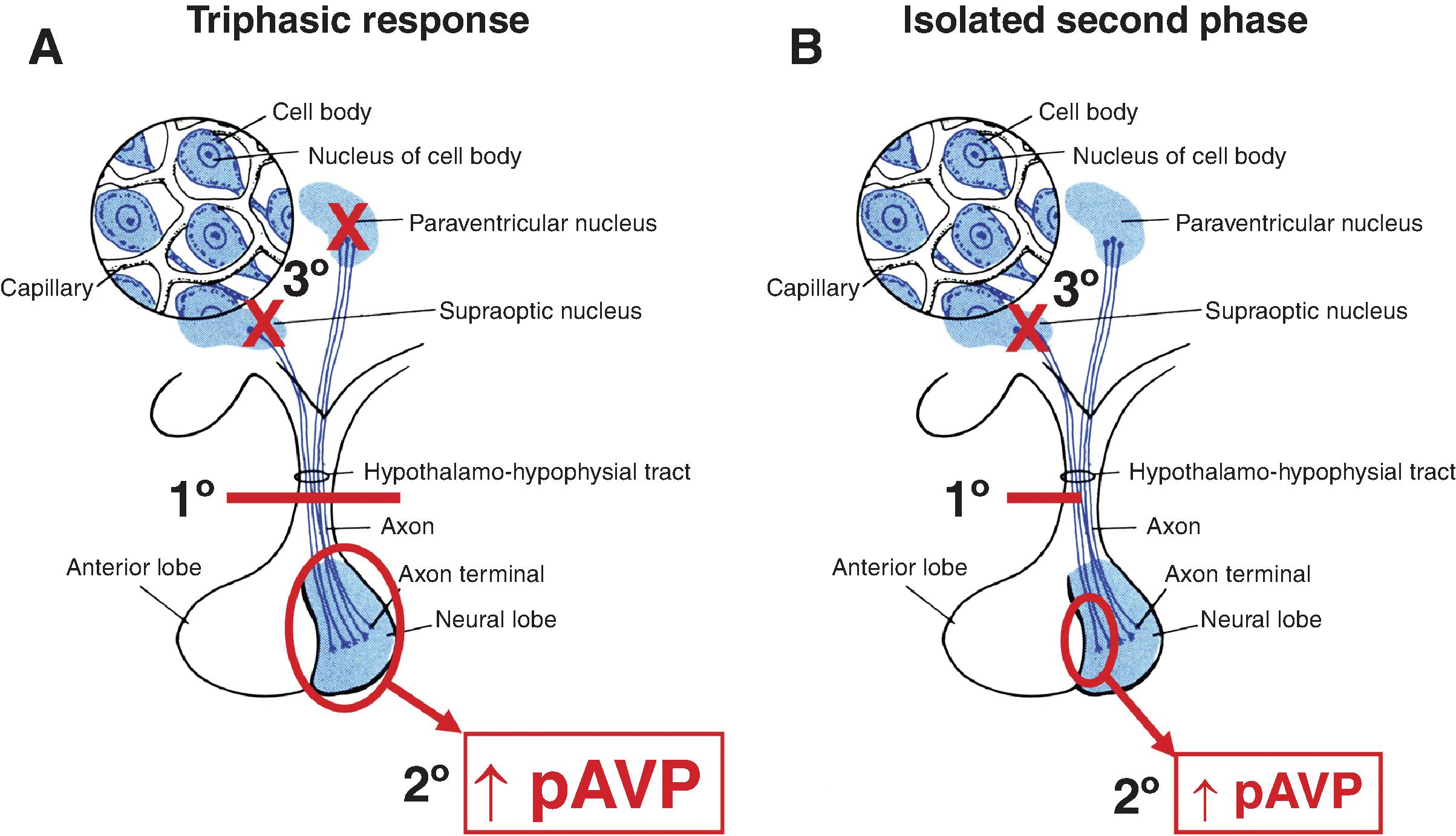
Hyponatremia is also a frequently encountered complication after transsphenoidal pituitary surgery, commonly with a delayed fall of serum [Na + ] toward the end of postoperative week 1. Given the potential delay in presentation of hyponatremia after surgery, this can present increased risk and complications for patients who are discharged early following surgery. Hyponatremia following transsphenoidal pituitary surgery has an incidence varying from 3% to 25%, depending on the study. , This hyponatremia is primarily due to SIADH; however, the presence of preoperative hypopituitarism makes postoperative hyponatremia more likely. The pathophysiology of SIADH in transsphenoidal pituitary surgery patients is due to the mechanical manipulation or irritation of the posterior pituitary or pituitary stalk. This pathology, responsible for fluctuating salt and water balance from the pituitary stalk injury, can be transient, triphasic, or permanent.
Hyponatremia following pituitary surgery or TBI occurs as part of the triphasic response, which is characterized by acute DI, followed by SIADH, and then DI again ( Fig. 18.1 A). The initial phase of the triphasic response consists of central DI (which can last 5 to 7 days) due to “stunning” of the AVP neurons and the severing of downstream nerve terminals in the posterior pituitary. Serum [Na + ] concentration increases at this time, and patients experience hypotonic polyuria and polydipsia if their thirst mechanism remains intact. Intravenous fluids are started during this phase in order to keep up with urinary losses and prevent or improve hypernatremia. The second phase (SIADH, which can last 2 to 14 days) results in hyponatremia due to the unregulated release of AVP either from the remaining degenerating neurons in the hypothalamus or from the remaining nerve terminals in the posterior pituitary. This phase of SIADH should be treated by fluid restriction. Given the vastly different management between the first two phases of the triphasic response (intravenous fluid administration versus fluid restriction), judicious monitoring of the patient’s urine output and serum [Na + ] concentration during all stages is pertinent in order to not paradoxically worsen the patients’ hyponatremia. The third and final phase of the triphasic response results again in DI due to the release of the remaining AVP from the posterior pituitary gland and the inability of the hypothalamus to produce more AVP. The major determinant of whether postoperative DI after transection of the pituitary stalk is transient or permanent depends to the level of the lesion: the closer the lesion is to the cell bodies of the AVP neurons in the hypothalamus, the more likely it is that the cell bodies will degenerate and permanent DI will ensue.