Time of onset
Early hemorrhage (≤24 h after the end of the index operation)
Late hemorrhage (>24 h after the end of the index operation)
Location
Intraluminal (intraenteric, e.g., anastomotic suture line at stomach or duodenum, or pancreatic surface at anastomosis, stress ulcer, pseudoaneurysm)
Extraluminal (extraenteric, bleeding into the abdominal cavity, e.g., from arterial or venous vessels, diffuse bleeding from resection area, anastomosis suture lines, pseudoaneurysm)
Severity of hemorrhage
Mild
Small or medium volume blood loss (from drains, nasogastric tube, or on ultrasonography, decrease in hemoglobin concentration <3 g/dl)
Mild clinical impairment of the patient, no therapeutic consequence, or at most the need for noninvasive treatment with volume resuscitation or blood transfusions (2–3 units packed cells within 24 h of end of operation or 1–3 units if later than 24 h after operation)
No need for reoperation or interventional angiographic embolization; endoscopic treatment of anastomotic bleeding may occur provided the other conditions apply
Severe
Large volume blood loss (drop of hemoglobin level by ≥3 g/dl)
Clinically significant impairment (e.g., tachycardia, hypotension, oliguria, hypovolemic shock), need for blood transfusion (>3 units packed cells)
Need for invasive treatment (interventional angiographic embolization, or re-laparotomy)
Table 32.2
International Study Group of Pancreatic Surgery (ISGPS) classification of postpancreatectomy hemorrhage: clinical condition and diagnostic and therapeutic consequences [5]
Grade | Time of onset, location, severity, and clinical impact of bleeding | Clinical condition | Diagnostic consequence | Therapeutic consequence | |
|---|---|---|---|---|---|
A | Early, intra- or extraluminal, mild | Well | Observation, blood count, ultrasonography, and, if necessary, computed tomography | No | |
B | Early, intra- or extraluminal, severe | Late, intra- or extraluminal, milda | Often well/intermediate, very rarely life-threatening | Observation, blood count, ultrasonography, computed tomography, angiography, endoscopyb | Transfusion of fluid, intermediate care unit (or ICU), therapeutic endoscopyb, embolization, re-laparotomy for early PPH |
C | Late, intra- or extraluminal, severe | Severely impaired, life-threatening | Angiography, computed tomography, endoscopyb | Localization of bleeding, angiography and embolization, (endoscopyb) or re-laparotomy, ICU | |
Time of onset (I). PPH is differentiated into early- and late-onset hemorrhage. If the onset of PPH is ≤24 h after the index operation, the PPH is classified into early PPH. Early PPH is usually caused by a technical failure of hemostasis during the initial operation or an underlying coagulopathy. Late-onset PPH (>24 h after the index operation) is associated with erosion of a peripancreatic vessel secondary to pancreatic fistula and arterial pseudoaneurysms [2, 5].
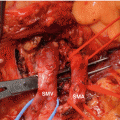
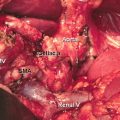
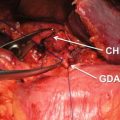
Location and cause (II). PPH may arise from the various sites: (a) arterial or venous, (b) suture lines of the anastomoses (e.g., gastroenteric, duodenoenteric, jejunojejunal, or pancreatico-enteric), (c) areas of resection (e.g., pancreas stump, retroperitoneum), (d) gastric/duodenal ulcer or diffuse gastritis, (e) eroded and ruptured pseudoaneurysm, or (f) hemobilia from previously placed endobiliary stents. In addition, PPH can be grouped into intraluminal and extraluminal according to the definite location. Peripancreatic vascular structures that may be the source of PPH are the stump of the gastroduodenal artery, splenic artery, branches of the superior mesenteric artery (SMA), the splenic vein stump, or, rarely, an intrapancreatic artery (Figs. 32.1 and 32.2) [5–7].
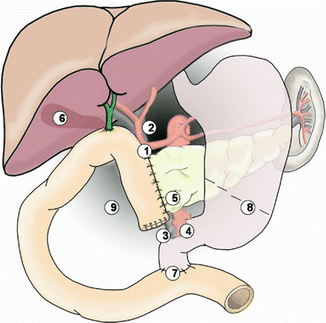
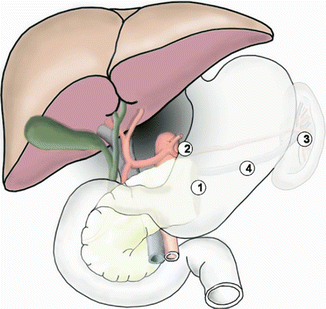

Fig. 32.1
Some potential bleeding sites after right-sided pancreatic resections. 1 Stump of gastroduodenal artery. 2 Tributaries of the portal vein and branches from the hepatic artery. 3 Tributaries of the superior mesenteric vein, including uncinate vessels. 4 Branches of the superior mesenteric artery, including jejunal mesenteric arterial branches to the left and inferior pancreaticoduodenal artery to the right. 5 Pancreatic cut surface and suture line of the pancreaticojejunostomy (PJ) site. 6 Gallbladder fossa after cholecystectomy. 7 Suture line of the duodenojejunostomy after pylorus-preserving pancreaticoduodenectomy. 8 Suture line of the gastrojejunostomy after classical pancreaticoduodenectomy. 9 Area of resection (retroperitoneum) (Reproduced from Wente et al. [5])

Fig. 32.2
Some potential bleeding sites after left pancreatic resection. 1 Pancreatic stump. 2 Tributaries of the splenic artery. 3 Splenic hilus (in case of spleen preservation)/tributaries of the splenic vein stump. 4 Area of resection (Reproduced from Wente et al. [5])
Severity (III). The severity of bleeding can be differentiated into two categories based on the amount of blood loss or transfusion requirements: (a) mild (no clinical impairment and transfusion requirements less than three units of packed cells) or (b) severe (more than four or six units of packed cells transfused within 24 h, a decrease in hemoglobin of more than 4 g/dl, or need for re-laparotomy or interventional angiographic therapy due to severe blood loss).
To summarize the ISGPS classification of PPH based on three main parameters (time of onset, location, and severity of hemorrhage): (1) onset is either early (≤24 h after end of the index operation) or late (>24 h); (2) location is either intraluminal or extraluminal; (3) severity of bleeding may be mild or severe (Table 32.1).
ISGPS also established a clinical grading system with three different grades of PPH (grades A, B, and C) considering the cumulative overall risk and clinical severity of the hemorrhage (Table 32.2). PPH grade A results only in a temporary and marginal variation of the standard postoperative course of the patient after pancreatectomy. In general, PPH grade A has no major clinical impact, and its occurrence should not be associated with a major delay of the patient’s hospital discharge. PPH grade B requires adjustment of a given clinical pathway, including further diagnostics and intervention; this grade of PPH will lead to therapeutic consequences such as the need for transfusion, the readmission to an intermediate or intensive care unit, and potential invasive therapeutic interventions, such as re-laparotomy or embolization. Most likely, the occurrence of PPH grade B will prolong the patient’s hospital stay. PPH grade C leads to severe impairment of the patient and should always be considered potentially life-threatening. Immediate diagnostic and therapeutic consequences are mandatory and often needed. The hospital stay of this group of patients is always prolonged and sometimes necessitates that the patient stays longer in the intensive care unit [5].
32.3 Incidence and Mortality
The incidence of PPH is 2–12%. PPH occurs in 4–16% of cases after a PD, in 1–3% of cases after a distal pancreatectomy (DP), and in 6% of cases after enucleation [8–15]. The mortality rate is 6–34% in the case of PPH grade B or C, and the PPH is the leading cause of mortality following PD and accounts for 11–38% of overall mortality [2, 5, 6, 12, 16–19].
Grutzmann et al. [6] reported the incidence and mortality rates according to the ISGPS definition; PPH occurred in 1.7% (grade B) and 4.0% (grade C) of total 945 patients who underwent pancreatic surgery, respectively. They also reported that one (6.2%) mortality in PPH grade B and 13 (34.2%) mortalities in PPH grade C. In our center, 42 (2.2%) patients of total 1,905 patients who underwent PD experienced the delayed arterial hemorrhage between 1995 and 2012. And 12 (28.6%) patients of 42 patients died during admission period. Choi et al. reported 22 cases of delayed hemorrhage after PD, of which the bleeding site could be verified by surgery or angiography in 17 patients. The sites of bleeding in 14 patients with arterial bleeding were five gastroduodenal artery stumps, three common hepatic arteries, three branches of SMA, one proper hepatic artery, one right hepatic artery, and one short gastric artery bleeding [14].
32.4 Diagnosis
PPH becomes apparent due to one or more of the following signs: blood loss through abdominal drains or nasogastric tube, hematemesis or melena, clinical deterioration of the patient, unexplained hypotension or tachycardia, or laboratory findings such as a decreasing hemoglobin concentration. Sentinel bleeding is a small amount of blood loss via abdominal drains or nasogastric tube several hours before massive hemorrhage, may be present (30–100%); recognizing this event as a sentinel bleed in a timely fashion may prevent severe and fatal outcomes.
32.4.1 Diagnosis of Early-Onset PPH
Early PPH is usually the result of technical failure during the index operation and can be divided into extraluminal bleeding into the abdominal cavity and intraluminal bleeding into the gastrointestinal tract. Various shortcomings during the index operation might lead to early postoperative hemorrhage irrespective of the potential site of the bleeding such as wide distances between successive suture lines, incomplete trans-fixation sutures, or slippage of ligatures. In addition, postoperative relief of vasospasm in smaller blood vessels, which remain undetected as potential bleeding sites during the operation, should be taken into account. Sometimes, an upper digestive hemorrhage originating from a gastric submucosal vessel can benefit from endoscopic hemostasis, but most surgeons would have some concerns about performing an early endoscopy after a pancreatic resection with an anastomosis in situ. They may require additional surgery to maintain hemostasis [2, 8, 21].
32.4.2 Diagnosis of Late-Onset PPH
Late PPH is often associated with POPF or biliary fistula. Accumulation or erosive damage to the vessels leads to pseudoaneurysm formation and may present as sudden hypotension or massive bleeding. If patients with suspected hemorrhage were hemodynamically stable, computed tomography (CT) angiography is the first diagnostic option. And then in case that arterial hemorrhage is suspected or pseudoaneurysm is detected, radiologic intervention should be performed. However, if the patient is hemodynamically unstable, diagnostic and therapeutic angiography should be performed immediately. The therapeutic angiographic techniques categorized into three groups: selective embolization, distal to proximal embolization, and stent graft insertion [8, 20–28].
32.4.2.1 CT
Contrast-enhanced CT scan of the abdomen can provide information regarding pseudoaneurysms, intra-abdominal fluid collections, and abscesses much more reliably than a ultrasonography (US) examination. Hence, it is the investigation of choice in hemodynamically stable patients, especially if features of sepsis are also present. However, if the size of pseudoaneurysm is small, it can be missed especially in the presence of inflammation. Therefore, a strong index of suspicion is important and should prepare for an emergency angiography even if the CT scan is negative.
32.4.2.2 Angiography
Angiography can make an accurate diagnosis of the site and cause of hemorrhage and resolve the PPH at the same time even in a hemodynamically stable patient. Angiographic evaluation should include the celiac axis and the superior mesenteric artery and their branches. In the presence of pseudoaneurysm, angiography gives a positive result even if active bleeding is not present. However, angiography may give false negative occasionally, because the pattern of delayed hemorrhage is intermittent and the amount is small in early stage. Therefore, early diagnostic angiography is recommended after sentinel hemorrhage occurs. Moreover, the utility of angiography is also dependent on the cause of bleeding. If the bleeding focus is the disrupted suture lines, angiography may give a negative result even in the presence of ongoing ooze.
32.4.3 Sentinel Bleeding
Sentinel bleeding was described by Brodsky and Turnbull in 1991 [29]. By ISGPS definition, sentinel bleeding is defined as a small amount of blood loss before massive hemorrhage via abdominal drains or nasogastric tube. Especially, if a patient with pancreatic fistula had sentinel bleeding, a diagnostic angiography may be necessary. Tien et al. reported that sentinel bleed was detected in 20 (7.1%) patients of 283 patients who underwent PD. In seven (35.0%) patients of 20 patients, angiography detected pseudoaneurysm. Therefore, prompt angiography should be performed for every detected sentinel bleed after PD [22]. However, sentinel hemorrhage is not necessarily present in all patients with delayed hemorrhage.
Tien et al. found sentinel hemorrhage in only three of ten patients, and Rumstadt et al. reported it in only 3 of 11 patients with delayed hemorrhage. In our center, 22 (52.4%) of 42 patients with delayed arterial hemorrhage patients represented sentinel bleeding signs. Sentinel bleeding group had lower mortality rate than without sentinel bleeding group (22.7% vs 35.0%) [3, 30]. Not all sentinel hemorrhage will go on to have massive bleeding. The exact natural history of sentinel hemorrhage remains unknown in view of the limited information available. De Castro et al. reported 11 patients with delayed massive hemorrhage after PD. They reported that nine patients had sentinel hemorrhage prior to massive bleed, but there were four other patients who had sentinel hemorrhage that was not followed by major bleeding [2].
Late-onset PPH sometimes appears as bleeding in two stages, with initial minimal bleeding that stops spontaneously (sentinel bleeding) followed by a significant recurrence of the hemorrhage associated with shock. In cases of sentinel bleeding, contrast-enhanced CT scanning is recommended to make an early diagnosis of a pseudoaneurysm and the usually associated complication of abdominal infection. The CT scan should be followed by specific treatment of the abdominal complication along with an angiography to provide endovascular treatment, which has an efficacy of approximately 80% [21, 22]. Radiological hemostasis can be obtained through the use of either coils or covered stents, allowing for the treatment of pseudoaneurysm without a collar [8, 20, 23–28].
32.5 Treatment
32.5.1 Initial Assessment
The early diagnosis and appropriate management of bleeding are essential to reduce the mortality rate. Continuous and close postoperative observation of the patient is mandatory to detect PPH immediately. Tachycardia and low blood pressure are reliable bedside indicators of progressive hypovolemia associated with significant PPH and should put the nursing and surgical team on high alert. Additionally, persistent bloody aspirate from a nasogastric tube or melena is a certain sign of intraluminal bleeding, and the volume of aspirate should be monitored at short and regular intervals since its primary detection. Furthermore, an assessment of the intra-abdominal drainage output, both in terms of quantity and quality, is critical to reach a clinical, bedside decision whether the hemorrhage is only intraluminal, extraluminal, or both, as can happen when anastomotic suture line bleeding results in anastomotic disruption with extravasation of blood into the peritoneal cavity. In these situations, all laboratory tests are mandatory on an emergency basis. They serve to correct hypovolemia, coagulation abnormalities, and electrolyte disturbances associated with major hemorrhage.
Rapid decision-making is essential when bleeding stigmata such as pseudoaneurysm on CT and sentinel bleeding are noted. Prompt operation for early bleeding and angiographic embolization for late bleeding are recommended.
32.5.2 Management of Early PPH
Signs of progressive abdominal distension and blood gushing from intra-abdominal drains are an indication for immediate re-laparotomy to identify and control the bleeding source. As a general rule, early severe hemorrhage is managed with re-exploration, as a surgically correctable source of bleeding is likely to be found. The best treatment option remains controversial, and surgery is usually considered the first-line treatment. This is especially true for early bleeding (i.e., occurring less than 24 h after the end of the surgical procedure); because early hemorrhage usually results from incomplete bleeding control, it is treated by reoperation (Fig. 32.3) [20, 23, 31, 32].
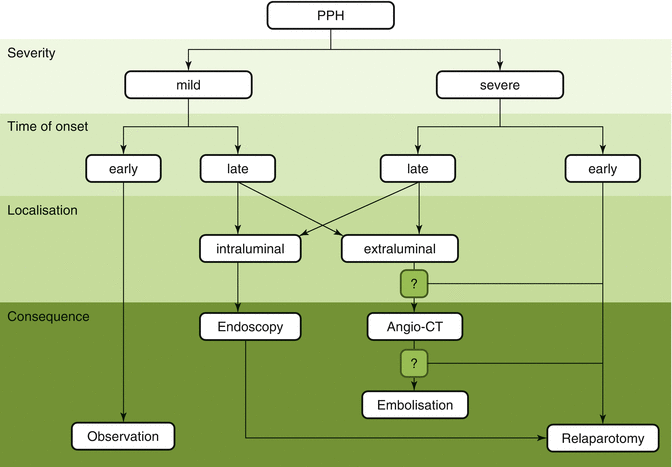

Fig. 32.3
Proposed algorithm for diagnosis and treatment of PPH (Reproduced from Grutzmann et al. [6])
The gastroduodenal artery, inferior pancreaticoduodenal artery, splenic artery, and the superior mesenteric and portal veins are the main sources of major intraperitoneal PPH. If the clinical condition is not stabilized after conservative management, re-laparotomy should be undertaken immediately. If massive bleeding is anticipated, blood clot removal should be performed carefully. The surgeon could identify the bleeding site though an abdominal wash with warm saline and careful suctioning. The surgical team has to examine carefully for all the potential sites of rebleeding and place drains to detect any rebleeding that may occur later on [33].
32.5.3 Management of Late PPH
32.5.3.1 Interventional Radiology
Recently, the recommended management of late PPH has been changed from surgery to radiological intervention, such as transarterial micro-coil embolization or the use of a stent graft (Fig. 32.3). Endovascular embolization of the hepatic artery trunk can be securely performed only if blood flow to the liver by an alternate route is confirmed. To reduce mortality of PPH patients, it is necessary to prevent other complications associated with pancreatic fistula following hemostasis. Proactive surgical intervention such as abscess drainage or remnant pancreatectomy is a key consideration [24–28, 31, 34–38].
The technique of transarterial intervention has achieved remarkable development in recent years due to the availability of a variety of fine angiography catheters that allow selective and even super-selective catheterization. Transarterial target artery embolization has an 83–100% hemostasis success rate [28, 39]. The liver has dual blood supply system from the right and left hepatic artery and portal vein. There are also collateral vessels. However, embolization of hepatic artery has a risk of hepatic ischemia. When the collateral flow is not sufficient, severe ischemia of the liver has been reported to lead to liver failure and even death. Liver abscesses may occur after embolization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree