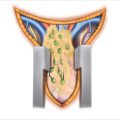
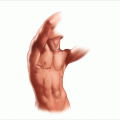Figure 11.1
(a) Prone positioning for posterior adrenalectomy with the table flexed; the dotted line designates the skin incision. (b) The skin incision is made vertically from the 10th rib and curved slightly towards the iliac crest
11.2 Needs and Indications
Indications for adrenal resection have been consistent across all eras of adrenal surgery. Neoplasms of the adrenal cortex are categorized as either functional or nonfunctional. Aldosterone-producing tumors leading to Conn’s syndrome (resulting in hypertension with significant hypokalemia) involve unilateral adrenal adenoma in 70–75% of patients. Surgery is indicated on the basis of imaging plus adrenal vein sampling to identify precisely the unilateral source of the excess aldosterone. Aldosteronomas are uniformly benign and small, almost never exceeding the size of 3 cm. Unilateral primary adrenal Cushing’s syndrome is due to either adrenocortical adenomas or adrenocortical carcinomas. Larger lesions with associated Cushing’s syndrome have a higher tendency for malignancy. Patients are identified by signs and symptoms of excess cortisol, with high 24 h urinary free cortisol and suppressed ACTH. Primary pigmented nodular adrenal disease and adrenal macronodular hyperplasia are rare familial causes of bilateral primary adrenal Cushing’s syndrome and can be approached by bilateral adrenalectomy. Patients with pituitary Cushing’s disease who have failed one or more transsphenoidal resections of their pituitary gland, with or without salvage attempts by gamma knife radiation, may be eligible for bilateral adrenalectomy to control severe symptoms.
Nonfunctional adrenocortical neoplasms (or so-called adrenal incidentalomas) have become commonly identified by the widespread use of cross-sectional imaging studies. Their incidence increases with age; in general, between 3% and 5% of patients receiving CT or MRI scans have some adrenal nodular disease, as do up to 7% of elderly patients. Most of these lesions are small, and once it is proven by biochemical testing that they do not make catecholamines or steroid hormones, their management depends on the size of the lesion and its rate of growth in serial imaging studies. In the current laparoscopic era, an indication for surgery for a nonfunctional adrenal cortical neoplasm would be a cutoff size ≥4 cm, or significant growth over a short period on sequential scans. Because there is no true curative treatment for adrenocortical carcinoma other than surgical resection, vigilant follow-up and appropriate resection is essential in managing these lesions.
Tumors of the adrenal medulla (pheochromocytomas) uniformly produce some level of catecholamine products, which can be measured by plasma catecholamines and metanephrines, or by urinary catecholamines, metanephrines, and vanillylmandelic acid (VMA). These patients are at risk for hypertensive crises and consequences such as myocardial infarctions, acute hypertensive cardiomyopathy, or cerebral vascular events. Any suitable surgical candidate with a biochemically identified pheochromocytoma should undergo adrenalectomy for resection of this potentially lethal lesion after appropriate preoperative alpha blockade.
Prior to the introduction of either laparoscopic transabdominal adrenalectomy or retroperitoneoscopic posterior adrenalectomy, the open posterior approach to the adrenal gland was preferred for small, functional lesions [2]. These lesions included aldosteronomas, small adrenocortical neoplasms causing Cushing’s syndrome, and even small lesions with metastatic disease. This approach is ideally suited for bilateral adrenalectomy of normal or modestly hypertrophied glands in the setting of failed treatment of pituitary Cushing’s, because these patients have a great degree of truncal obesity and positioning allows a fairly straightforward bilateral approach. Limitations of posterior adrenalectomy relate to larger tumor size. The posterior approach leaves a fairly narrow window of view, so tumors generally larger than 5–6 cm are not easily accessed by this exposure. Because virtually all lesions smaller than 5–6 cm can be successfully removed by either transabdominal laparoscopic adrenalectomy or retroperitoneoscopic posterior video-assisted adrenalectomy, the posterior approach to the adrenal should be viewed somewhat as a historical operation. There are rare indications for utilizing this approach today, however, despite the availability of other, minimally invasive techniques. For example, the use of video-assisted techniques may be quite challenging in patients who are very morbidly obese due to pituitary Cushing’s, because of their body habitus.
11.3 Surgical Technique
In 1936, Young [3] initially described the posterior approach for the resection of the adrenal glands. Patients are positioned completely prone on the operating room table with their arms stretched over their heads (Fig. 11.1a). Rolls are placed under the pelvis and under the chest/axillary area with a gap between the padding to allow the intra-abdominal contents to hang forward and pull away from the retroperitoneum. All pressure points are padded, with care taken to provide padding for the face area with the endotracheal tube.
Incisions are made in a hockey-stick manner, as described by Young (Fig. 11.1b). In the prone position, one can palpate the spinous processes of the lumbar vertebrae even in obese patients and count the ribs. The 10th, 11th, and 12th ribs should be identified by deep palpation and marked with a pen. Attempts should be made to palpate the paraspinus muscles, specifically the sacrospinalis muscle, as the optimal position of the vertical portion of the incision is just on the lateral border of that muscle, but it may not be identifiable in obese patients with a considerable adipose layer. One should make a vertical incision from the 10th intercostal space along the lateral border of the sacrospinalis muscle, approximately four fingerbreadths lateral to the spinous processes. When extending the incision inferiorly below the 12th rib, the incision should be gently angled laterally towards the posterior superior iliac spine.
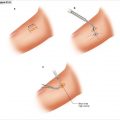
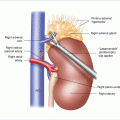
The incision is carried sharply down to the fascial part of the latissimus dorsi muscle, which lies under this hockey-stick incision (Fig. 11.2). At the superior aspect, one may have to divide some muscle fibers and then, with the subcutaneous tissue parted again, feel the sacrospinalis muscle. Incise to the deep lumbar fascia just on the lateral border of the sacrospinalis muscle. The sacrospinalis muscle is retracted medially and the origins of the 12th and 11th ribs are palpated (Fig. 11.3a). Once the 12th rib is identified, the soft tissue is opened down to the periosteum (Fig. 11.3b), which is then stripped with an elevator. This rib is resected all the way to the edge of the spine. Attempts can be made to preserve the neurovascular bundle at the inferior margin of the 12th rib, or at least the nerve, as division will lead to numbness and laxity of the flank muscles. In patients of short stature and often in Cushing’s patients with considerable truncal obesity, the 11th rib may need to be excised in a similar manner. If two ribs are excised, then the neurovascular bundle and 12th rib must be divided, which will result in numbness along the dermatomal distribution of that nerve root. With this exposure, the anterior lumbar dorsal fascia is incised and the retroperitoneal space is identified and visualized, with variable degrees of spongy adipose tissue (Fig. 11.4). This initial incision into the lumbar dorsal fascia is made towards the inferior portion of the incision, to try to avoid entering the chest cavity. With gentle blunt dissection, one should next identify the diaphragm and gently reflect the pleura cephalad (Fig. 11.5).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree