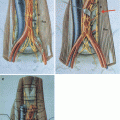
Fig. 9.1
(a) Encasement of the infrarenal aorta by mature teratoma (b) extensive and meticulous preparation of the major retroperitoneal vessel: the left renal vein is marked with a blue vessel loop, the renal arteries are prepared and the infrarenal aorta is marked with a red vessel loop. (c) infrarenal aorta has been resected and replaced by an aortic graft
9.2 PC-RPLND in Small Residual Lesions
In patients with residual lesions <1 cm, the role of PC-RPLND is discussed controversially based on the finding that up to 20 and 8 % of patients will harbour mature teratoma and vital cancer. However, this approach has been challenged by recent retrospective studies from three groups. Kollmannsberger et al. [4] analysed 276 patients who underwent systemic chemotherapy for metastatic NSGCT. One hundred sixty-one (58.3 %) achieved a complete remission (residual lesions <1 cm), and all patients were followed without surgical resection. After a mean follow-up of 40 (2–128) months, relapses were observed in 6 %, and none of them died after appropriate salvage therapy. Ninety-four percent of the patients belonged to the IGCCCG good prognosis group. Ehrlich et al. [5] evaluated 141 patients who were observed after systemic chemotherapy and had residual lesions <1 cm. After a mean follow-up of up to 15 years, 9 % of the patients relapsed and 3 % of the patients died due to testis cancer. IGCCCG risk group classification predicted the outcome best: recurrence-free survival and cancer-specific survival were 95 and 99 %, respectively, in men who belonged to the good risk group, whereas it dropped to 91 and 73 % in the intermediate and poor risk group. The German Testicular Cancer Study Group (GTCSG) analysed the outcome of 392 patients who underwent PC-RPLND for residual lesions of any size [6]. 9.4 and 21.8 % of the men with residual lesions <1 cm harboured vital cancer and mature teratoma, respectively. These numbers increased to 21 and 25 % in patients with residual lesions of 1–1.5 cm and to 36 and 42 % in men with lesions larger than 1.5 cm.
Based on these data, PC-RPLND for small residual masses might only be indicated in patients with (1) intermediate or poor prognosis at initiation of chemotherapy and/or (2) >50 % teratoma in the orchiectomy specimen. The remainder can be managed conservatively with close follow-up.
9.3 Resection of Extraretroperitoneal Disease
In patients with residual masses at multiple sites, an individual decision should be made regarding the number and extension of resections based on the risk of relapse and on quality-of-life issues [1–3]. Resection of residual tumours outside the abdomen or lung should also be considered on an individual basis, since discordant histology is found in 35–50 % of patients. Pulmonary or mediastinal residual masses harbour necrosis/fibrosis in 90 % if the retroperitoneal masses did not contain mature teratoma or viable cancer [7, 8]. Management of liver lesions by postchemotherapy retroperitoneal lymph node dissection must be individualised. Observation may be warranted for liver lesions requiring complicated hepatic surgery regardless of retroperitoneal pathology [9]. The concordance between retroperitoneal and liver histology was 49 % overall, including 94 % for necrosis, 26 % for teratoma and 36 % for cancer. Liver necrosis alone was found in 94, 70 and 50 % of patients with retroperitoneal necrosis, teratoma and cancer, respectively.
9.3.1 Considerations for the Most Appropriate Surgical Strategy
PC-RPLND requires detailed knowledge of the retroperitoneal anatomy, familiarity with surgical techniques of the vascular and intestinal structures, as well as profound experience in the management of patients with testicular cancer [2, 3]. Depending on the size and the extent of the residual lesions, the surgeon has to modify his surgical approach to the retroperitoneal space. An abdominal midline incision can be used in most patients with unilateral and infrahilar disease, whereas a Chevron incision might be more suitable in those men with bilateral and suprahilar disease. Retrocrural disease is best approached by a thoracoabdominal incision.
9.3.2 Special Preoperative Imaging Studies
Imaging studies should allow an adequate assessment of the large retroperitoneal vascular structures since involvement of the inferior vena cava (IVC) and the abdominal aorta can be expected in about 6–10 % and 2 %, respectively [2, 3, 10]. Magnetic resonance imaging represents the most appropriate imaging technique to predict infiltrations of the vessel wall and the presence of an intracaval tumour thrombus. Infiltrations of the IVC wall or IVC thrombi should be completely resected since about two thirds of the patients harbour vital cancer or mature teratoma in the infiltrating masses. The necessity for aortic replacement is rare and usually accompanied by large residual masses involving additional adjacent structures and making additional surgical procedures necessary (Fig. 9.1).
9.4 Timing of PC-RPLND
PC-RPLND should be initiated within 6–12 weeks after chemotherapy. Hendry et al. retrospectively analysed the outcome of 443 patients undergoing either immediate or elective PC-RPLND once progression of the residual masses was demonstrated [11]. A significant benefit with regard to progression-free survival (83 % versus 62 %, p = 0.001) and cancer-specific survival (89 % versus 56 %, p = 0.001) was identified for the immediate surgical approach.
9.4.1 Extent of PC-RPLND
Early retrospective and single-centre studies indicate that a modified PC-RPLND might be a safe approach in men with limited retroperitoneal disease and right/left primary tumours with no evidence of teratoma or viable cancer on frozen section analysis [2, 3]. However, application of the modified unilateral template to PC-RPLND still is discussed controversially based on the 3–8 % incidence of mature teratoma or viable cancer in the contralateral landing zone. Two experienced groups reported their experience on modified unilateral template PC-RPLND. The group at Indiana University has performed a limited PC-RPLND in 100 men with low-volume retroperitoneal disease (<5 cm) confined to the primary landing zone of the primary tumour [12]. After a mean follow-up of 32 months, only four patients relapsed, all outside the boundaries of the modified and even of the bilateral template. The 2- and 5-year disease-free survival was 95 %.
The GTCSG assessed the oncological necessity of full bilateral retroperitoneal PC-RPLND in 152 patients [13]. If patients exhibited a well-defined lesion ≤2 cm, modified PC-RPLND was performed; lesions >5 cm were always treated by a full bilateral PC-RPLND (Fig. 9.2). Lesions 2–5 cm in diameter were approached dependent on the site of the primary lesion and the location of the mass: interaortocaval residuals were always approached with a full bilateral PC-RPLND, whereas para-aortic and paracaval lesions were treated by a modified PC-RPLND if the metastatic site corresponded to the site of the primary lesion. There was a significant difference with regard to postoperative morbidity with more complications in patients undergoing extended surgery (p < 0.001). Antegrade ejaculation was preserved in 85 % of patients undergoing modified PC-RPLND, whereas it could not be preserved in 75 % of the cases undergoing full bilateral PC-RPLND. Eight (5.2 %) recurrences were observed after a mean follow-up of 39 (6–105) months: one in-field relapse following modified PC-RPLND and seven recurrences even outside the boundaries of full bilateral PC-RPLND. The two-year disease-free survival was 78.6 and 92.8 % for bilateral and modified PC-RPLND, respectively.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree