Fig. 11.1
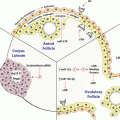
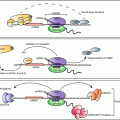
Calcium , phosphate , PTH, FGF23, 1,25D and FGF23 interactions. There are endocrinological feedback loops that govern mineral homeostasis. FGF23, fibroblast growth factor 23; PTH, parathyroid hormone , P, serum phosphate (Silver and Naveh-Many 2009)
Parathyroid cells have few secretory granules as compared to other endocrine cells and therefore PTH production is regulated largely at the levels of PTH gene expression and parathyroid cell proliferation (Habener et al. 1984). The changes in PTH gene expression by calcium phosphate and CKD are due to post-transcriptional mechanisms affecting PTH mRNA stability . The parathyroid also responds to changes in serum 1,25(OH)2 vitamin D (1,25D) which decreases PTH levels. PTH in turn, increases the renal synthesis of 1,25D. 1,25D then increases blood calcium largely by increasing the efficiency of intestinal calcium absorption (Fig. 11.1). The increased serum calcium would shut down PTH secretion by activating the parathyroid calcium sensing receptor (CaR) (Brown et al. 1993). In contrast to the post-transcriptional regulation of PTH gene expression by changes in serum calcium and phosphate levels and CKD, 1,25D decreases PTH gene transcription in vitro and in vivo (Silver et al. 1985, 1986; Russell et al. 1986). The 1,25D receptor (VDR) is expressed in the rat parathyroid, confirming that the parathyroid is indeed a target organ for vitamin D (Naveh-Many et al. 1990). Several groups have identified DNA sequences in the 5′-flanking region of the PTH gene that may mediate the negative regulation of PTH gene transcription by 1,25D (Demay et al. 1992; Fujiki et al. 2005; Murayama et al. 2004). The action of 1,25D to decrease serum PTH is used therapeutically in the management of CKD patients. They are given 1,25D or its prodrug 1α(OH)-vitamin D3 to treat or prevent the secondary hyperparathyroidism (2HPT) of CKD. Fibroblast growth factor 23 (FGF23) is a bone-derived phosphaturic hormone that acts on the kidney to increase phosphate excretion and suppress biosynthesis of 1,25D (Fig. 11.1). FGF23 signals through the fibroblast growth factor receptor 1 (FGFR1) bound by the transmembrane protein Klotho (Kurosu et al. 2006). We have identified the parathyroid as a target organ for FGF23 (Ben Dov et al. 2007a). Recombinant FGF23 decreases PTH gene expression and secretion in vivo and in vitro by activation of the MAP kinase pathway (Fig. 11.1). In CKD there are very high levels of serum FGF23 together with increased serum PTH levels, due to down regulation of the kloth-FGFR1 FGF23 receptor complex in the parathyroid, leading to resistance of the parathyroid to FGF23 (Galitzer et al. 2010; Komaba et al. 2010). In this chapter we discuss the molecular mechanisms of the post-transcriptional regulation of PTH gene expression by calcium, phosphate and CKD.
2 Post-transcriptional Regulation of PTH Gene Expression by Calcium , Phosphate and CKD
Hypocalcemia, hypophosphatemia and CKD regulate PTH mRNA stability by post-transcriptional mechanisms. Dietary induced hypocalcemia and experimental CKD markedly increase PTH secretion, mRNA levels and after prolonged stimulation, parathyroid cell proliferation (Moallem et al. 1998; Naveh-Many et al. 1995). In the rat, hypocalcemia leads to a >10-fold increase in PTH mRNA levels and this increase is post-transcriptional affecting mRNA stability (Moallem et al. 1998). Serum phosphate also has a direct effect on PTH secretion, PTH mRNA levels and parathyroid cell proliferation (Kilav et al. 1995; Naveh-Many et al. 1995). Careful in vivo studies showed that the regulation of PTH gene expression by dietary induced hypophosphatemia is independent of changes in serum calcium and 1,25D (Fig. 11.1) (Kilav et al. 1995). In vitro studies, when tissue architecture was maintained, confirmed the direct effect of phosphate on the parathyroid (Almaden et al. 1996, 1998; Nielsen et al. 1996; Rodriguez et al. 1996; Slatopolsky et al. 1996). In vivo studies showed that phosphorus depletion leads to a dramatic decrease in rat PTH mRNA levels and that this effect is post-transcriptional as is the effect of hypocalcemia to increase PTH mRNA levels (Kilav et al. 1995; Moallem et al. 1998). There is a ~60-fold difference in PTH mRNA levels between hypocalcemic and hypophosphatemic rats and these dietary models were used as tools to define the mechanism of the post-transcriptional regulation of PTH gene expression (Moallem et al. 1998; Nechama et al. 2008).
Secondary hyperparathyroidism is a common disorder in patients with CKD and in experimental models where there are increases in PTH gene expression, secretion and parathyroid hyperplasia (Naveh-Many and Silver 1990; Silver et al. 2002). In CKD patients with 2HPT, calcimimetics and oral phosphate binders are effective drugs used to control the high serum PTH levels (Block et al. 2004; D’Haese et al. 2003; Joy and Finn 2003; Moe et al. 2005). In a rat model of CKD induced by an adenine high phosphorus diet, PTH mRNA levels were increased already after 7 days of the diet and more so at 21 days (Levi et al. 2006). The addition of the calcimimetic R568 or an oral phosphate binder, lanthanum carbonate (La), decreased PTH mRNA and serum PTH levels in the CKD rats (Ben Dov et al. 2007b). The effects of CKD and the calcimimetic or the phosphorus binder were post-transcriptional as were those of changes in serum calcium and phosphate (Moallem et al. 1998; Nechama et al. 2009a; Yalcindag et al. 1999).
Therefore, PTH gene expression is regulated post-transcriptionally by serum calcium , phosphate , CKD and its management by calcimimetics and oral phosphorus binders. The changes in PTH mRNA stability are mediated by protein-PTH mRNA interactions that determine the susceptibility of PTH mRNA to the degradation machinery (Kilav et al. 1995; Levi et al. 2006; Moallem et al. 1998; Nechama et al. 2008). The balanced interaction of stabilizing and destabilizing proteins with the PTH mRNA determines PTH mRNA stability and levels, serum PTH and the resultant response of the parathyroid to calcium, phosphate and CKD (Nechama et al. 2008, 2009c).
3 The PTH mRNA cis-Acting Protein Binding Element
For many mRNAs, post-transcriptional regulation involves critical cis-acting elements, mostly in the untranslated regions (UTR ) that are targets for trans-acting proteins regulating mRNA stability and translation (Barreau et al. 2006). Adenine and Uridine-rich elements (ARE ) are a well-defined family of cis-acting elements critical for the expression of many unstable mRNAs that code for cytokines, transcription factors, proto-oncogenes and other mRNAs (Fig. 11.2) (Brewer 2002). Three classes of AREs have been identified, two of which contain several scattered or overlapping copies of the pentanucleotide AUUUA, while class III AREs lack this motif but contain A and U rich sequences and possibly other unknown determinants. A number of ARE binding protein s (ARBPs) have been identified. K-homology splicing regulator protein (KSRP ) is an examples for decay promoting factors (Barreau et al. 2006; Gherzi et al. 2004). KSRP interacts with the large multiprotein 3′–5′ exoribonuclease complex, the exosome, and recruits it to target ARE containing mRNAs thereby promoting their rapid degradation (Chou et al. 2006; Linker et al. 2005). Other ARE binding proteins, such as the ELAV protein family members (mainly HuR ), are stabilizing factors and AU rich binding factor 1 (AUF-1) promotes either decay or stabilization, depending on the mRNA and cell type (Fig. 11.2) (Wilusz and Wilusz 2004).
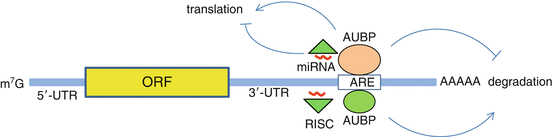

Fig. 11.2
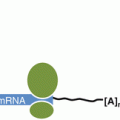
Regulation of mRNA fate. mRNAa are comprised of the 5′ methyl cap (57G), open reading frame (ORF), 5′ and 3′-untranslated regions (UTRs ) and poly A tail. Following transcription, Adenylate/uridylate (AU) rich binding proteins (ARBPs) bind to the mRNA and determine mRNA stability , localization and translation. AU-rich elements (AREs) within the mRNA 3′-UTR act to stabilize or destabilize the mRNA. Stabilized RNA undergoes translation in ribosomes, whereas destabilized RNA undergoes deadenylation , decapping , and degradation by the exo- or endo-nucleases. microRNAs (miRNAs ) as part of the RNA-induced silencing complex (RISC ), bind to target mRNAs at their 3′-UTR and target them for translational inhibition or degradation
PTH mRNAs are typical eukaryotic mRNAs that contain a 7-methylguanosine cap at the 5′ terminus and a poly adenylic nucleic acid (poly A) stretch at the 3′ terminus (Naveh-Many 2005; Kemper 1986). PTH mRNA consists of three exons coding for the 5′-untranslated region (5′-UTR ) (exon I), the prepro region (exon II) and the structural PTH hormone together with the 3′-UTR (exon III) (Fig. 11.3a). The PTH mRNA 3′-UTR in all species is rich in A and U nucleotides (Bell et al. 2005b).
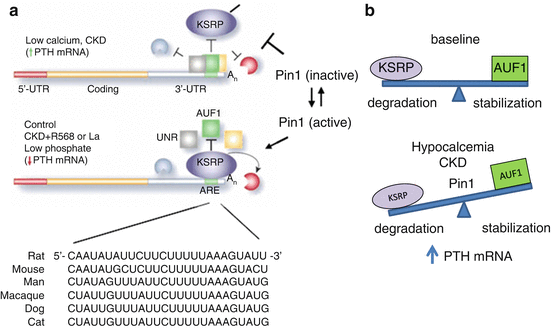

Fig. 11.3
Model for the regulation of PTH mRNA stability by changes in calcium and phosphate levels and CKD. a. Schematic representation of the PTH mRNA including the 5′-UTR (red), coding region (orange) the 3′-UTR (gray) and the 26 nucleotide cis acting AU rich element (ARE ) (green). The nucleotide sequence of the element in different species is shown. Nucleotides that differ from the rat sequence are in bold. In hypocalcemic rats and in rats with experimental chronic renal failure (CKD) there is increased PTH mRNA stability and levels and this is associated with decreased binding of the destabilizing protein KSRP and increased binding of the protective proteins, AUF1 (AU rich binding factor) and Unr (Up-stream of N-ras), to the PTH mRNA 3′-UTR ARE. In control and more so in rats fed a low Pi diet, or treated with a calcimimetic (R568) or a phosphate binder (La) there is increased interaction of PTH mRNA with KSRP and decreased interaction with AUF1. KSRP recruits the exosome to PTH mRNA leading to PTH mRNA decay . The peptidyl prolyl cis/trans isomerase Pin1 is upstream of KSRP and leads to KSRP dephosphorylation and activation thus increasing KSRP-PTH mRNA interaction and mRNA degradation . In hypocalcemia or renal failure, Pin1 is inactive and KSRP is phosphorylated and hence less potent to bind PTH mRNA. AUF1 then binds the PTH mRNA 3′-UTR ARE with a greater affinity leading to increased PTH mRNA stability. Reproduced from Nechama et al. (2009c) and Silver and Naveh-Many (2009). b. Model for ARE-directed PTH mRNA decay. Basal PTH levels are obtained when the mRNA destabilizing ARBPs, such as KSRP and the stabilizing proteins such as AUF1, provide a balance of mRNA degradation and stabilization. In hypocalcemia or CKD, Pin1 is less active, resulting in KSRP phosphorylation and decreased binding to PTH mRNA, shifting the balance to mRNA stabilization
Protein-PTH mRNA binding experiments demonstrated specific interaction of rat and human parathyroid extracts with transcripts for the rat and human PTH mRNA 3′-UTR terminal region. This binding was regulated by calcium or phosphorus depletion and correlated with PTH mRNA levels and stability in vivo (Moallem et al. 1998). A 26 nucleotide ARE at the 3′ end of the PTH mRNA 3′-UTR is the minimal protein binding region and is highly conserved in the PTH mRNA 3′-UTRs of rat, mouse, man, dog and cat (Fig. 11.3a). The conservation of sequences within a region that does not code for protein (UTR) suggests that the binding element is a functional unit that has been evolutionarily conserved (Bell et al. 2005a; Kilav et al. 2001). This PTH mRNA 3′-UTR element is a cis-acting type III ARE that determines PTH mRNA stability and its regulation by changes in serum calcium, phosphate and CKD (see below) (Kilav et al. 2001; Nechama et al. 2008).
There is no parathyroid cell line, therefore a cell-free mRNA in vitro degradation assay (IVDA) was utilized to identify the factors involved in PTH mRNA decay . In the IVDA, extracts from parathyroids were incubated with in vitro transcribed RNA probes for the PTH mRNA to estimate mRNA decay mediated by the parathyroid extracts. IVDA has been shown to reproduce differences in mRNA stability that occur in vivo and specifically the differences in PTH mRNA stability induced by calcium , phosphate and CKD (Fritz et al. 2000; Moallem et al. 1998; Nechama et al. 2008). Parathyroid extracts from hypocalcemic and CKD rats stabilized and extracts from hypophosphatemic rats destabilized transcripts for the PTH mRNA compared to parathyroid extracts from control rat, correlating with steady state PTH mRNA levels in vivo. These effects were dependent upon the terminal 60 nucleotides of the PTH mRNA 3′-UTR that contain the ARE (Moallem et al. 1998; Nechama et al. 2009a; Yalcindag et al. 1999). A 63 nucleotide transcript containing the conserved 26 nucleotide ARE and flanking regions was both necessary and sufficient to regulate PTH mRNA stability and to confer responsiveness of reporter mRNAs to changes in calcium and phosphate (Kilav et al. 2001). Structural analysis showed that the PTH mRNA 3′-UTR and in particular the ARE is dominated by significant open regions with little folded base pairing (Kilav et al. 2004).
4 The PTH mRNA trans Acting Stabilizing Proteins
Two PTH mRNA binding and stabilizing proteins have been identified by PTH RNA affinity chromatography. These trans acting proteins are AUF1 and Up-stream of N-ras (Unr) (Dinur et al. 2006; Sela-Brown et al. 2000). AUF1 consists of four isoforms (p37, p40, p42, and p45) that are generated by alternative pre-mRNA splicing of AUF1 mRNA (Wagner et al. 1998). These proteins bind the PTH mRNA 3′-UTR and are part of the PTH mRNA-parathyroid protein binding complex. Addition of recombinant AUF1 isoforms to parathyroid extracts from phosphorus depleted rats prevented the rapid degradation of PTH transcripts in IVDAs (Sela-Brown et al. 2000). Interestingly, calcium and phosphorus depletion as well as kidney failure lead to post-translational modifications of the PTH mRNA stabilizing protein AUF1, with no change in AUF1 protein levels (Bell et al. 2005c; Levi et al. 2006). These modifications may lead to the differences in AUF1-PTH mRNA binding affinity and PTH mRNA stability . Over-expression of AUF1 in human embryonic kidney (HEK) 293 cells co-transfected with expression plasmids for the PTH gene or a chimeric GH gene containing the PTH mRNA 3′-UTR 63 nucleotide cis-acting element, stabilized PTH mRNA and chimeric reporter mRNA, but not a PTH mRNA lacking the cis element nor a reporter mRNA containing a truncated PTH element (Bell et al. 2005a; Dinur et al. 2006). Unr over-expression had a similar effect. Knock-down of all four AUF1 isoforms or Unr by siRNA led to the opposite effect, decreasing PTH mRNA levels (Bell et al. 2005c; Dinur et al. 2006). AUF1 also stabilized a reporter mRNA containing the bovine PTH mRNA protein-binding element that is different from the one characterized in the rat (Bell et al. 2005a). These studies identified AUF1 and Unr as PTH mRNA stabilizing proteins (Fig. 11.3). However, the half-life of mRNAs is determined by the coordinate association of both stabilizing and destabilizing factors with the specific mRNA in the cytoplasm (Fig. 11.3b).
5 The PTH mRNA trans Acting Destabilizing Protein KSRP
The decay promoting protein KSRP was identified as a PTH mRNA trans acting destabilizing protein. KSRP is a RNA-binding protein implicated in a variety of cellular processes, including transcription, alternative pre-mRNA splicing, and editing as well as mRNA localization and stability (Gherzi et al. 2004; Lellek et al. 2000; Min et al. 1997; Snee et al. 2002). KSRP binds to ARE containing mRNA and promotes rapid mRNA decay of several inherently labile mRNAs, recruiting the 3′ to 5′ exoribonucleolitic complex exosome to the RNAs (Gherzi et al. 2004). The central part of the KSRP contains 4 adjacent K homology (KH) domains that are required for AREs recognition and interaction with the mRNA degradation machinery to promote decay of target mRNAs (Gherzi et al. 2004).
KSRP binds the PTH mRNA 3′-UTR ARE both in vivo in the parathyroid glands and in vitro in transfected cells (Nechama et al. 2008). This binding is decreased in glands from calcium -depleted or experimental CKD rats in which PTH mRNA is more stable compared to parathyroid glands from control and phosphorus-depleted rats in which PTH mRNA is less stable. KSRP also interacts with the PTH mRNA 3′-UTR ARE in vitro in transfected cells. The KH domains 3–4 of KSRP were sufficient for this association, as reported for other mRNAs (Gherzi et al. 2004; Nechama et al. 2008). KSRP over-expression and knock down experiments showed that KSRP decreased co-transfected PTH mRNA steady-state levels through the PTH mRNA ARE. Overexpression of KSRP specifically decreased both rat and human PTH mRNA levels in cotransfected HEK293 cells and accelerated PTH mRNA decay . This effect of KSRP was dependent on the PTH mRNA ARE. Conversely, KSRP knock-down increased both PTH mRNA stability and PTH mRNA steady-state levels. Moreover, PTH mRNA decay was dependent on the KSRP-recruited exosome in parathyroid extracts (Nechama et al. 2008). By its interaction with PTH mRNA ARE, KSRP would recruit the exosome to degrade PTH mRNA. These findings suggested that KSRP-PTH mRNA interactions control PTH mRNA t1/2 by recruitment of a degradation complex to PTH mRNA. AUF1 binding to the same region in the PTH mRNA 3′-UTR competes with the binding of KSRP and thus protect PTH mRNA from degradation (Fig. 11.3).
Of interest, KSRP interacts with the endoribonuclease polysomal ribonuclease 1 (PMR1) (Chernokalskaya et al. 1998; Nechama et al. 2009b). PMR1 facilitates PTH mRNA degradation , adding an additional layer of complexity to the regulation of PTH mRNA stability . PMR1 mediated decrease in PTH mRNA levels involves the PTH mRNA 3′-UTR ARE , KSRP and the exosome. KSRP recruits a degradation complex, comprising both exo- and endo-ribonucleases to PTH mRNA, thus controlling its mRNA half-life (Nechama et al. 2009b).
6 The Balanced Interactions of Stabilizing AUF1 and Destabilizing KSRP Determine PTH mRNA Stability and Levels
The pattern of interactions of KSRP and AUF1 with PTH mRNA suggest that these proteins have opposing roles in the regulation of PTH gene expression in vivo (Fig. 11.3). KSRP and AUF1 protein-PTH mRNA interactions in the parathyroid were studied using a RNA immunoprecipitation (RIP) assay which provides a snap-shot of protein-mRNA interactions at a specific time point in vivo. In this assay the parathyroid glands were cross-linked, AUF1 or KSRP containing complexes immunoprecipitated and the amount of PTH mRNA associated with each of the proteins determined by qRT-PCR analysis. KSRP-PTH mRNA interaction was decreased in glands from calcium depleted or CKD rats, where PTH mRNA is more stable, and increased in parathyroids from phosphorus depleted rats, where PTH mRNA is less stable. In contrast, AUF1-PTH mRNA interactions were increased by hypocalcemia and CKD and decreased in the phosphorus depleted rat parathyroids (Nechama et al. 2008). Both proteins bind to the same cis ARE in the PTH mRNA 3′-UTR (Fig. 11.3a). Consequently, the differential interactions of KSRP and AUF1 suggest that these proteins compete for their binding to the PTH mRNA 3′-UTR element, having antagonistic effects on PTH mRNA stability (Fig. 11.3) (Naveh-Many and Nechama 2007). Indeed, in vitro, over-expression of the PTH mRNA stabilizing protein AUF1 isoform p45 blocked KSRP-PTH mRNA binding and partially prevented the KSRP mediated decrease in PTH mRNA levels (Nechama et al. 2008). Therefore, the balanced interaction of these proteins with the PTH mRNA 3′-UTR determines basal PTH mRNA levels and the regulation of PTH mRNA levels (Fig. 11.3b).
Calcimimetics and oral phosphorus binders that are widely used to treat secondary hyperparathyroidism decrease PTH mRNA levels post-transcriptionally in CKD rats (Nechama et al. 2009a). KSRP -PTH mRNA interaction was increased by both the calcimimetic R568 and the oral phosphorus binder La which decreased PTH mRNA levels. IVDAs showed that PTH mRNA is destabilized by parathyroid extracts from CKD rats treated with R568 or La compared to parathyroid extracts from untreated CKD rats. This destabilizing effect of R568 and La was dependent upon KSRP and the PTH mRNA 3′-UTR . Therefore, the calcimimetic R568 and correction of serum phosphate by La determine PTH mRNA stability through KSRP-mediated PTH mRNA decay , thereby decreasing PTH expression (Nechama et al. 2009a). Changes in binding of the trans acting factors to the PTH mRNA therefore determine PTH gene expression in CKD and after management of the 2HP of CKD by both calcimimetics or oral phosphorus binders as well as after changes in calcium and phosphate (Fig. 11.3) (Nechama et al. 2009a).
6.1 The Peptidyl-Prolyl Isomerase Pin1 Determines PTH mRNA Levels and Stability in Secondary Hyperparathyroidism
The above results indicate that KSRP and AUF1 directly or indirectly respond to changes in serum calcium and phosphate concentrations and CKD by altering their association with PTH mRNA leading to differences in PTH mRNA stability and levels (Nechama et al. 2008, 2009a). These changes could be a result of post-translational modifications of these ARE binding protein s , affecting their binding affinity to the PTH mRNA. Indeed, as stated above, AUF1 is post-translationally modified in the parathyroids of 2HPT rats and this is at least in part due to differences in protein phosphorylation (Bell et al. 2005c; Levi et al. 2006). KSRP is also a phospho-protein with two identified phosphorylation sites at serine 193 (S193) and threonine residue 692 (T692). Phosphorylation at these sites prevents KSRP association with the ribonuclease degradation complex exosome (S193) or compromises KSRP binding to ARE-containing target mRNAs (T692) and hence their decay (Gherzi et al. 2006; Ruggiero et al. 2007). Therefore, the differential interaction of KSRP and AUF1 with PTH mRNA after changes in serum calcium and phosphate may involve KSRP and AUF1 post-translational modifications.
The peptidyl prolyl cis/trans isomerase Pin1 specifically binds phosphorylated serine/threonine-proline protein motifs and catalyzes the cis/trans isomerization of the peptide bonds thereby changing the biological activity, phosphorylation and turn-over of its target proteins (Wulf et al. 2002; Zhou et al. 2000). Pin1-catalysed conformational regulation has a profound impact on many key proteins involved in various cell functions (Winkler et al. 2000; Lu et al. 1999). Pin1 was shown to regulate the turnover of ARE containing mRNAs, mainly cytokine mRNAs, through the interaction and isomerization of ARE binding protein s . Pin1 interacts with AUF1 and thereby stabilizes both GM-CSF and TGFβ mRNAs (Shen et al. 2005, 2008). These observations led us to speculate that Pin1 may be involved in PTH gene expression through AUF1 and/or KSRP interaction and isomerization. Indeed, Pin1 is a PTH mRNA destabilizing protein in vivo and in vitro (Nechama et al. 2009c). The regulation of PTH mRNA stability by Pin1 was mediated by the PTH mRNA 3′-UTR ARE and by the mRNA destabilizing protein KSRP. We showed for the first time that KSRP is a Pin1 target protein. Pin1 interacts with phosphorylated KSRP at S181, leading to KSRP dephosphorylation and activation. Importantly, Pin1 enzymatic activity was decreased in parathyroid extracts from rats with 2HPT due to either a calcium depleted diet or CKD. Pharmacological inhibition of Pin1 increased PTH mRNA levels and stability and decreased KSRP-PTH mRNA interaction in the parathyroid. This decreased interaction would increase PTH gene expression after Pin1 inhibition. Furthermore, Pin1 −/− mice display increased serum PTH and PTH mRNA levels. Therefore, Pin1 determines basal PTH expression in vivo and in vitro and decreased Pin1 activity correlates with increased PTH mRNA levels in rats with 2HPT. These results demonstrate that Pin1 is a key mediator of PTH mRNA stability and indicate a role for Pin1 in the pathogenesis of the 2HPT of CKD (Nechama et al. 2009c). Our data suggest that phosphorylated KSRP at S181 is inactive. Upon interaction with Pin1, cis–trans isomerization of the proline bond in KSRP leads to conformational change, exposing the phosphorylated S181 residue and possibly additional phosphorylation sites. This leads to KSRP dephosphorylation by a still unidentified phosphatase. KSRP then interacts with PTH mRNA and enhances its decay. A low calcium diet and CKD lead to decreased Pin1 isomerase activity in the parathyroids of these rats. This decreased Pin1 activity would prevent KSRP dephosphorylation, resulting in decreased KSRP-PTH mRNA interaction, inhibition of PTH mRNA degradation , and increased PTH mRNA levels (Fig. 11.3a). The trigger for the reduced Pin1 activity in the parathyroid glands of 2HPT rats is not known. Post-translational modifications of Pin1 protein itself may play a role in this regulation. Pin1 is post-translationally modified by phosphorylation that affects its ability to interact with target proteins and its activity. PKA mediated phosphorylation at Ser residue 16 affects the interaction of Pin1 with its target proteins. Phosphorylation at Ser residue 71 by the protein kinase DAPK1, inhibits Pin1 isomerization activity (Lu et al. 2002; Lee et al. 2011). Future studies may identify the factors that decrease Pin1 activity in the hyper-functioning parathyroid glands of 2HPT. Another main challenge will be to unravel the cell signaling cascade and the particular kinases responsible for KSRP phosphorylation at S181 that determine PTH mRNA-KSRP interactions and PTH mRNA decay .
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree