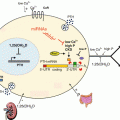
Fig. 3.1
The role of cytokines in mediating communication between immune cells. Transcripts in grey are cytokine transcripts that contain AREs in their 3′UTRs . Transcripts labeled with an asterisk (*) contain GREs in their 3′UTRs. Arrows indicate direct interactions and/or activations. Blunt-ended lines indicate inhibitory effects. Reproduced from Vlasova-St. Louis and Bohjanen (2014)
A variety of ARE -containing transcripts interact with many different ARE-binding proteins, resulting in complex patterns of post-transcriptional regulation. The destabilizing functions of different AREs, and their ability to interact with different ARE-binding proteins are not equivalent. The sequence characteristics and decay patterns of different AREs allowed them to be categorized into classes based on their sequence features and decay kinetics (Chen and Shyu 1995). A more extensive database of ARE-containing transcripts from mouse, rat and man has been generated using bioinformatics (Bakheet et al. 2003, 2006; Halees et al. 2008). This database groups AREs into five clusters depending on the number of overlapping AUUUA pentamers within the 3′UTR of a transcript with clusters 1–5 having five to one overlapping AUUUA pentamers, respectively. Cluster I AREs are enriched in secreted proteins, such as cytokines, and are involved in the growth of hematopoietic and immune cells. The other ARE clusters are found within a diverse set of transcripts. In total, ARE-containing transcripts compose approximately 5–8 % of the human transcriptome (Bakheet et al. 2001). AREs play decisive roles in regulating the effects of cytokines on inflammatory responses since mutation of the ARE in cytokine genes such as TNF-alpha or IFN-gamma resulted in profound autoimmune-like inflammatory syndrome (Hodge et al. 2014; Jacob et al. 1991).
AREs regulate mRNA decay by interacting with ARE -binding proteins that can either function to stabilize or destabilize the bound transcript. Numerous ARE-binding proteins involved in the regulation of mRNA turnover have been described in a variety of cell types (reviewed in Gratacos and Brewer 2010; Li et al. 2012; Von Roretz et al. 2011). Typically, mRNA molecules move through different cellular compartments within messenger ribonucleoprotein (mRNP ) complexes, dynamically associating with RNA-binding proteins (RBPs) that bind to conserved cis-elements found in subsets of transcripts (Turner and Hodson 2012a, b). The association of specific RBPs with subsets of transcripts containing conserved regulatory cis-elements coordinates the fate of these bound transcripts through post-transcriptional processes such as translation, intracellular localization, storage or mRNA decay (reviewed in Blackinton and Keene 2014; Keene 2007). Following cellular activation, the set of transcripts bound by a given RBP can be used to define post-transcriptional regulatory networks. RNA-immunoprecipitation (RNA-IP) experiments, in which RNA-binding proteins are immunoprecipitated from cellular lysates using an antibody against a given RBP, and co-purified mRNAs are identified, provided evidence that such networks of coordinately regulated RNAs exist. Target transcripts of several ARE-binding proteins, including HNRNPD (Wu et al. 2013), ZFP36 (Emmons et al. 2008; Stoecklin et al. 2008), ELAVL1 (Fan et al. 2011; Lopez De Silanes et al. 2004b; Mukherjee et al. 2009), KSRP (Winzen et al. 2007) and TIA-1 (Lopez De Silanes et al. 2005) were identified using RNA-IP techniques, and these targets represent distinct but overlapping sets of transcripts. ARE-binding protein targets include transcripts encoding cytokines and cytokine signaling components. Integration of the effects of multiple ARE-binding proteins likely decides the fate of ARE-containing transcripts (Mansfield and Keene 2009).
Several ARE -binding proteins, including ZFP36 (also known as TTP ), BRF1, BRF2, KSRP , and HNRNPD (AUF1 ) destabilize transcripts by recruiting cellular deadenylases and enzymes involved in both 5′→3′ and 3′→5′ mRNA decay processes, whereas other ARE-binding proteins, such as ELAVL1 (also known as HuR ) and ELAVL4, stabilize target transcripts by preventing deadenylation and decay (Bronicki and Jasmin 2013; Brooks and Blackshear 2013; Khabar 2010; Sarkar et al. 2011; White et al. 2013; Wu et al. 2013). ARE-containing mRNA can also be degraded by ribonucleases, such as ZC3H12A, which initiates decay by cleaving the stem loop mRNA structure in the near proximity of an ARE. Examples of ZC3H12A targets are TNF-alpha, IL1b, IL6 and IL12b, IL2, c-Rel and Ox40 transcripts (Matsushita et al. 2009; Uehata et al. 2013), which are important regulators of lymphocyte activation. These same ARE-containing transcripts are also targets of ELAVL1 (Fan et al. 2011; Lopez De Silanes et al. 2004b; Mukherjee et al. 2009). ELAVL1 has been reported to stabilize transcripts of multiple proinflammatory cytokines including, TNF-alpha, VEGF , IL13, IL17 COX2, GATA3 and a number of chemokines through AREs in their 3′UTRs , often leading to increases in both mRNA and protein levels (Stellato et al. 2011; Winzen et al. 2004). The stabilization of ARE-containing transcripts does not always lead to increased protein expression, however. For example when ELAVL1 is in a complex with TIA-1 protein, TNF-alpha and COX2 mRNAs were stabilized but their translation did not increase (Katsanou et al. 2005).
ARE -containing transcripts can interact with different ARE-binding proteins during the course of an inflammatory response through competitive binding . For example, the ARE-binding proteins ELAVL1 and ZFP36 compete with one another for certain ARE-containing transcripts (Raghavan et al. 2001). More than 50 % of ZFP36 target sites in 3′UTRs also function as ELAVL1 target sites (Mukherjee et al. 2014). This potential for ZFP36 and ELAVL1 to compete for the same binding site on a single mRNA has implications for dynamic changes in binding during the course of adaptive immune responses. For example, shortly following T lymphocyte activation, cytoplasmic ELAVL1 levels increase transiently, stabilizing a network of ARE-containing transcripts and allowing their increased expression. Later in T cell activation, ZFP36 induction promotes the displacement of ELAVL1 from ARE-containing transcripts, allowing ZFP36 to mediate their rapid decay (Raghavan et al. 2001). This process allows the transient expression and subsequent degradation of ARE-containing transcripts during the course of immune responses. Cellular signals can also alter the functions of ARE-binding proteins over the course of innate immune responses. ZFP36 activity is regulated through its phosphorylation by p38 MAPK-activated protein kinase 2 (p38/MK2), following lipopolysaccharide (LPS) stimulation of macrophages. This phosphorylation of ZFP36 promotes its association with 14-3-3 protein and prevents ZFP36 from recruiting 3′ deadenylases to the bound transcript (Sun et al. 2007). Thus, through LPS-mediated phosphorylation of ZFP36, ARE-containing transcripts encoding inflammatory mediators such as CCL3, IL1, IL6, IL17, and COX2 are induced by virtue of transcript stabilization and thereby promote inflammation (Kang et al. 2011; Lee et al. 2012; Ronkina et al. 2010). As the immune response resolves, ZFP36 is dephosphorylated by the phosphatase PP2A, allowing ZFP36 to return to its baseline function, promoting the degradation of transcripts encoding these pro-inflammatory mediators (Frasca et al. 2010; Sun et al. 2007).
Understanding the role of AREs in balancing the expression of pro-inflammatory cytokines during inflammatory responses has led to the development of new anti-inflammatory therapies. For example, the novel drug, aganirsen, functions as an anti-inflammatory agent by impairing 14-3-3b-ZFP36 complex formation, preventing the stabilization and upregulation of ARE -containing cytokines, including IL8, TNF-alpha, IL21-beta, IL12, and IL22, during inflammatory responses (Colin et al. 2014). Thus, understanding ARE-mediated regulation of cytokine gene expression may lead to the further development of anti-inflammatory agents. In summary, the coordinate regulation of networks of ARE-containing transcripts, including cytokine networks, is a dynamic process following immune cell activation, which involves activation-induced expression of ARE-binding proteins, competition between ARE-binding proteins with opposing activities, and multiple phosphorylation events mediated through signaling pathways.
3 Regulation by GREs
Studies regarding AREs and ARE -binding proteins have led to identification of other cis-elements and proteins that coordinately regulate mRNA decay (Vlasova and Bohjanen 2008). For example, bioinformatic analysis of short-lived transcripts expressed in primary human T cells led to the identification of the sequence, UGUUUGUUUGU (known as a GRE), to be highly enriched in the 3′UTR of transcripts that exhibited rapid degradation (Vlasova et al. 2008). Introduction of the GRE into the 3′UTR of a beta-globin reporter transcript induced rapid decay of the otherwise stable beta-globin transcript, demonstrating that the GRE functions as a mediator of mRNA degradation . The GRE binds to the protein CELF1, leading to the decay of GRE-containing transcripts. Knockdown of CELF1 in HeLa cells led to stabilization of GRE-containing reporter transcripts, further implicating CELF1 as a mediator of GRE-dependent mRNA degradation (Rattenbacher et al. 2010; Vlasova et al. 2008). A database of GRE-containing transcripts (Halees et al. 2011) was generated by categorizing GREs into five clusters based on the number of overlapping GUUUG pentamers found in the 3′UTR of a transcript, with clusters 1–5 having five to one overlapping GUUUG pentamers, respectively. These GRE-containing transcripts encode a variety of proteins with diverse biological functions including cellular signaling, growth, development and apoptosis regulation. Our recent analysis of CELF1 revealed that targets contained not only the GRE sequence UGUUUGUUUGU but also a GU-repeat sequence which could bind CELF1 and mediate mRNA decay. Based on these findings, the GRE sequence definition was revised to UGU[G/U]UGU[G/U]UGU (Rattenbacher et al. 2010).
CELF1 binds numerous target transcripts involved in cytokine signaling pathways (Vlasova-St. Louis and Bohjanen 2011, 2014; Vlasova-St. Louis et al. 2013). Genome-wide RNA-IP experiments in HeLa cells (Rattenbacher et al. 2010), primary human T cells (Beisang et al. 2012), and mouse myoblasts (Lee et al. 2010), identified hundreds of CELF1 target transcripts. Comparison of molecular functions of the CELF1 bound transcripts among the different cell types revealed enrichment of mRNAs encoding regulators of transcription, post-transcriptional control and cell cycle. In primary human resting T cells, CELF1 binds to and mediates the degradation of numerous transcripts involved in cellular activation and proliferation, presumably to maintain the cell in a quiescent state. Rapid changes in the expression of these GRE-containing transcripts occur immediately following activation of T lymphocytes. T cell receptor-mediated activation promotes the phosphorylation of CELF1, inhibiting its ability to bind to GRE-containing transcripts (Beisang et al. 2012). The lack of CELF1 binding to mRNA within first 24 h following T cell stimulation correlates with a transient increase in expression of GRE-containing transcripts involved in cellular activation and proliferation. Thus, CELF1 functions in resting T cells to mediate the degradation of GRE-containing transcripts that promote cell proliferation, and subsequent CELF1 phosphorylation following T cell activation prevents CELF1 binding to mRNA, leading to the stabilization and upregulation of GRE-containing transcripts (Vlasova-St. Louis and Bohjanen 2014).
Post-transcriptional networks are instrumental for cytokines to rapidly transmit intercellular signals to maintain homeostasis and direct the normal course of inflammatory responses. Post-transcriptional regulation through AREs impacts cytokine production by affecting the stability of cytokine transcripts. Numerous components of cytokine signaling, however, are regulated through GREs. Figures 3.2, 3.3, and 3.4 depict transcripts encoding signaling components downstream of various cytokine receptors whose steady state mRNA levels changed following immune activation. As can be seen, GRE-containing transcripts (grey transcripts) encode components of signaling pathways downstream from these receptors. Overall, these receptors transduce a broad range of intracellular signals that shift cell activation states, alter the rate of proliferation and production of other cytokines. These pathway analyses indicate that the GRE/CELF1 network plays important roles in regulating the dynamic expression of cytokine signaling components through mRNA degradation and thereby modulates the strength and duration of inflammatory responses.
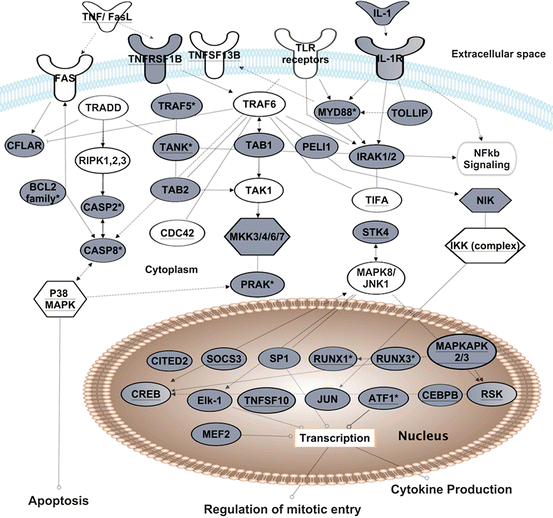
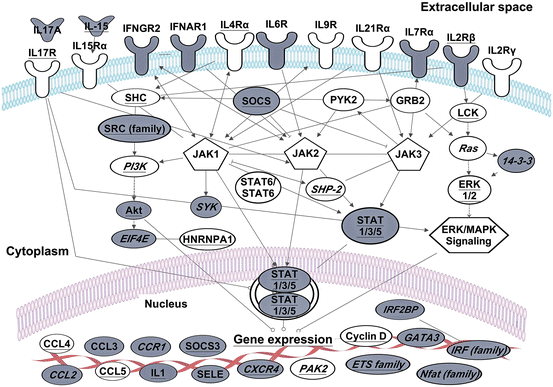
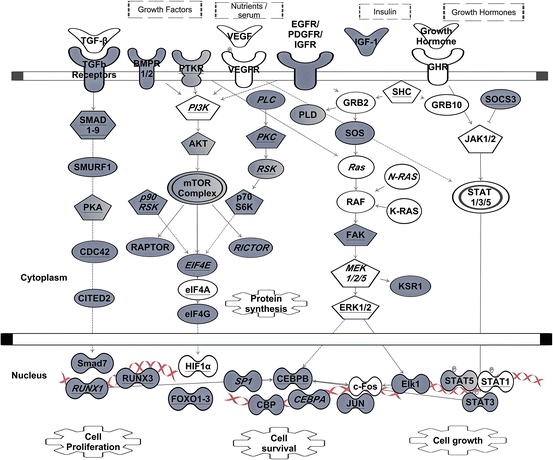

Fig. 3.2
The role of GREs and CELF1 in TNF, TLR or IL1 receptor signaling. Transcripts shown exhibited changes in steady-state levels following T cell receptor stimulation of primary human T cells (Raghavan et al. 2004). Transcripts in grey contain GREs. Transcripts with underlined text were identified as CELF1 target transcripts in human T cells by RNA-immunoprecipitation (Beisang et al. 2012). This figure is modified from (Vlasova-St Louis and Bohjanen 2014)

Fig. 3.3
The role of GREs and CELF1 in interferon and interleukin receptor signaling. Transcripts shown exhibited changes in steady-state levels following T cell receptor stimulation of primary human T cells (Raghavan et al. 2004). Transcripts in grey contain GREs. Transcripts with underlined text were identified as CELF1 target transcripts in human T cells by RNA-immunoprecipitation (Beisang et al. 2012). Transcripts marked with text in italics are also bound to ELAVL1 in activated cells (Mukherjee et al. 2009; Fan et al. 2011). This figure is modified from Vlasova-St. Louis and Bohjanen (2014)

Fig. 3.4
The role of GREs in growth factor signaling. Signals transmitted through tumor growth factor receptors (TGFR), vascular endothelial, epidermal, platelet-derived or insulin growth factor receptors (VEGFR, EGFR, IGFR, PTDGFR) are directed through several major pathways, such as SMAD, mTOR, RAS, PLC, or JAK/STAT. Proper expressions of these transcripts cooperatively contribute to the overall cellular signaling outcome (such as cell proliferation, cell survival and cell growth). Transcripts in grey contain GREs. Transcripts with underlined text were identified as CELF1 target transcripts in resting human T cells by RNA-immunoprecipitation (Beisang et al. 2012). Transcripts marked with text in italics are also bind ELAVL1 in activated cells (Mukherjee et al. 2009; Fan et al. 2011). Arrows indicate direct interactions and/or activations. Blunt-ended lines indicate inhibitory effects. This network diagram was built using Ingenuity Pathway Assistant Software
4 Cross-Talk between Networks
The network of ARE -containing transcripts encodes various cytokines (Fig. 3.1) and cytokine signaling molecules that depends on circuits of GRE-containing transcripts to function. In particular, GRE-containing transcripts encode diverse components of cytokine receptor signaling (Figs. 3.2, 3.3, and 3.4). Moreover, numerous GRE-containing CELF1 target transcripts are also targets of the ARE-binding protein ELAVL1, indicating a functional interaction and potential competition between these RNA-binding proteins. RNA recognition sequences for CELF1 require precise GU repeats or GUUU repeats, whereas the recognition sequence for ELAVL1 is less precise, and ELAVL1 binds to a variety of U-rich sequences, including GU-rich sequences or a poly U sequence. Thus, CELF1 and ELAVL1 may compete for binding to a subset of target transcripts. In contrast to ELAVL1, other ARE-binding proteins that recognize precise AUUU repeats, such as ZFP36, do not bind to GRE sequences and do not compete for binding sites. The comparison of CELF1 targets that were immunoprecipitated from normal T cells (Beisang et al. 2012) with ELAVL1 targets from HeLa (Lopez De Silanes et al. 2004a) or Jurkat cells (Mukherjee et al. 2009), showed a subset of transcripts involved in cytokine signaling that are targets of both proteins (Vlasova-St. Louis and Bohjanen 2014). Depending on which protein competitively binds to the GRE in the target transcript, dichotomous biochemical effects may result in either CELF1 mediating degradation or ELAVL1 mediating stabilization. We hypothesize that in resting cells these transcripts are bound to CELF1 and targeted for degradation, but in activated immune cells, CELF1 becomes phosphorylated and is rendered unable to bind to GRE sequences. ELAVL1 then binds to the GRE, displacing CELF1, stabilizing the transcripts, and allowing them to be translated. Later in the immune response, CELF1 becomes dephosphorylated and then displaces ELAVL1 to mediate transcript degradation. Although further evidence is needed, this model could explain the transient expression of certain GRE-containing transcripts following T cell activation.
The dynamic interplay of GRE and ARE networks appears to regulate nearly all known cytokine signaling pathways. For example, signals downstream of TNF-alpha receptors interact with signals downstream of FAS (also TNFRSF6) to regulate apoptosis. CELF1 regulates expression of a number of apoptotic proteins downstream of FAS receptor through destabilization of pro-apoptotic mRNAs, simultaneously affecting cell death rate and proliferation. CELF1 has been shown to co-immunoprecipitate with transcripts encoding TNF receptor super family members and other molecules downstream of TNF receptors (underlined transcripts in Fig. 3.2) (Beisang et al. 2012; Lee et al. 2010; Rattenbacher et al. 2010). Interestingly, CELF1 and ELAVL1 both bind to and affect the stability of TNF mRNA (Dean et al. 2001; Zhang et al. 2008) and other regulators including effector caspases and cytochrome C mRNA, which are activated downstream of TNF receptors involved in the regulation of apoptosis. Both CELF1 and ELAVL1 proteins regulate stability of transcripts encoding members of the BCL2 superfamily such as BCL2, BNIP3, BCL2L2, BAD and BAX, which modulate programmed cell death (Abdelmohsen et al. 2007; Beisang et al. 2012; Talwar et al. 2013). Thus, signaling through the TNF receptors is controlled by GRE-containing transcripts (also CELF1 targets), which cooperate with ELAVL1 target transcripts to regulate apoptosis.
As shown in Fig. 3.2, multiple GRE-containing transcripts encode signaling components downstream of the IL1 receptor. Proper signaling through this cytokine receptor requires coordination of ARE and GRE networks. Several IL1 receptor signaling components, including STK4, CDC42, MYD88, TIFA, MEF2, RSK, cyclin D have been shown to be ELAVL1 targets in RNA-IP experiments (Mukherjee et al. 2009). Many target transcripts, shared by CELF1 and ELAVL1, are transcription factors or early response genes such as RUNX1, JUN, myc or CREB that are transiently up-regulated following cellular activation and then down-regulated through rapid mRNA decay . Chronic imbalances in the turnover rates of these transcripts could promote onset and progression in diseases such as autoimmunity or cancer through aberrant immune cell activation, excess inflammation and inappropriate cell death (Candido and Hagemann 2013; Moudgil and Choubey 2011; Vlasova et al. 2005).
Many other interleukin and interferon receptors also utilize signaling proteins encoded by GRE-containing transcripts (Fig. 3.3), and rely on interplay between ARE and GRE networks to maintain homeostasis. Cytokines that bind to receptors containing the common gamma chain, such as IL2, IL4, IL7, IL9, IL15 and IL21, are pivotal for immune responses (Baker et al. 2007; Liao et al. 2011). These receptors activate Janus kinases (JAK1 and JAK2) to phosphorylate members of the STAT (Signal transducers and activators of transcription) family of transcription factors (Jenkins 2014). STAT proteins form hetero- or homodimers, and upon translocation into nucleus they induce the transcription of genes including cytokine, chemokine and adhesion molecule genes. One of the induced gene family, SOCS, plays a role in inhibiting JAK kinase activity and consequently, down-regulates immune activation (Linossi et al. 2013). Interestingly, SOCS3 mRNA is also a CELF1 and ELAVL1 target, perhaps to allow its expression only at the appropriate time during the resolution phase of the immune response. Engagement of interferons by interferon receptors also activates JAK-STAT, PI3K and MAPK signaling pathways (Khabar and Young 2007). Interferon family members of type I, II, and III contain functional AREs that allow precise post-transcriptional control by ARE-binding proteins. Interferon receptor mRNAs contain GREs and likely function to maintain the balance in cytokine-receptor signaling and ultimately the course of immune responses (Fig. 3.3). Thus, the induction and control of interferon responses requires coordination of ARE and GRE networks.
Growth factor-induced cell proliferation, adhesion, and migration often depend on ARE and GRE post-transcriptional regulatory networks. Transforming growth factor-beta (TGF-beta) signals are transduced by trans-membrane type I and type II serine/threonine kinase receptors (TGFR1/2), which are encoded by GRE- and ARE-containing mRNAs (Fig. 3.4). TGF-beta maintains tissue homeostasis by regulating the cellular proliferation rate, differentiation and survival. The activated TGF receptor complex induces oligomerization of several downstream SMAD protein family members. Activated SMADs regulate transcription of a number of GRE-containing mRNAs (ex. CITED2, CXCL2, NKX, CDKN1A, VEGF , IL3RA, TGFBR1, etc.), which in turn, become a subject of post-transcriptional regulation by CELF1 (or ELAVL1), once they leave the nucleus. In pathological conditions, TGF-beta signaling, by activating RAS oncogenic pathway, upregulates a large set of ARE-containing genes and specifically VEGF mRNA (Kanies et al. 2008), perhaps due in part to dysfunctional mRNA decay pathways. The RAS–PI3K–Akt kinase–mTOR pathway is a well-established signaling axis that modulates the proliferation and survival of many cell types through growth factor receptor signaling (reviewed elsewhere Bitterman and Polunovsky 2012; Cao et al. 2008; Weichhart and Saemann 2008). Growth factors are beneficial for tissue growth and regeneration, but abnormal growth factor signaling can fuel inflammation and metastases (Carmeliet and Jain 2011). Since ARE and GRE-containing transcripts encode important regulators of apoptosis and cell cycle downstream of growth factor receptors, transformed cells may usurp these post-transcriptional networks to their advantage as they become malignant (Fuxe and Karlsson 2012).
Numerous studies describe an important role of ELAVL1 in the pathogenesis of inflammatory diseases that involve over-production of inflammatory cytokines or diseases characterized by cytokine deficiency. Most studies support a role for ELAVL1 malfunction in the promotion of inflammation and proliferation in human diseases such as autoimmune diseases and cancer (Khabar 2010; Srikantan and Gorospe 2012). Furthermore, genetic deletion of ELAVL1 in thymocytes (Papadaki et al. 2009) and myelocytes (Yiakouvaki et al. 2012) predisposed mice to exaggerated inflammatory responses and inflammation-driven oncogenesis. In contrast, CELF1 functions as an inhibitor of a network of transcripts that promote cellular activation and proliferation (Beisang and Bohjanen 2012). The opposing effects of CELF1 and ELAVL1 may have important implications for new therapies for proliferative diseases such as cancer or autoimmunity. Thus, understanding the crosstalk of CELF1, ELAVL1 and other RBPs may uncover novel therapeutic strategies to fine-tune the balance in proliferative or proinflammatory pathways involved in human diseases. In the future, more work needs to be done to develop interventions targeting post-transcriptional mechanisms involved in inflammatory responses such as the severe reaction that occurs in sepsis, which involves self-amplifying networks of inflammatory mediators often referred to as ‘cytokine storm’.
5 Summary and Perspective
Post-transcriptional networks defined by AREs and GREs regulate cytokine production and signaling. The ARE and GRE networks represent distinct subsets of transcripts that work in concert to coordinate the function of cytokines over the course of an immune response. AREs are critical regulators of cytokine production and play important roles in cytokine signaling, but a distinct subset of cytokine signaling components, regulated by GREs, is needed for precise physiological function of cytokines. Thus, an effective immune response requires crosstalk between ARE and GRE pathways to appropriately regulate cytokine responses over time. Perhaps, integrative studies of interactions between GREs, AREs and RBPs as modulators of coordinated gene expression could be translated to novel treatment strategies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree