Introduction
The thyroid gland can be affected with benign and malignant diseases that will require thyroid surgery. About 150,000 thyroidectomies are performed each year in the United States. This common procedure has a low morbidity and mortality, especially when performed by intermediate- and high-volume surgeons compared with those who do less than three thyroidectomies per year. , However, injuries to the esophagus or trachea during thyroidectomy can have serious consequences, including death.
The advances in thyroid surgery have made this a safe operation. Compared to 1850 when the mortality rate was 40%, now it approaches 0%. Although death after a thyroidectomy is a rare event, it can still happen. Gomez-Ramirez in a study including 30,495 thyroidectomies from 26 different centers found 20 deaths. Most of the deaths were attributed to airway complications including tracheal injury and cervical hematoma causing respiratory compromise. Sepsis secondary to esophageal injury was also found as a cause of death in one patient of this cohort. It is important to mention that half of the mortalities in this series had an associated retrosternal goiter.
Morbidity after thyroidectomy is also low, with an overall complication rate between 3% and 5%. Common complications include wound infection (0.02% to 0.5%), hematoma (0.3% to 4.3%), transient recurrent laryngeal nerve palsy (1% to 2%), permanent recurrent laryngeal nerve palsy (less than 1%), transient hypoparathyroidism (1.6% to 50%), permanent hypoparathyroidism (0% to 13%), and chylous fistula (less than 1%).
Tracheal and esophageal injuries can also occur; however, the incidence is so low that only a few case reports and case series exist in the literature that can help us understand, diagnose, and treat these problems. Most of the reports agree that this complication is so infrequent that surgeons would at most encounter one case during their entire career. Nonetheless, it is important to be aware of these complications, including their diagnosis and treatment.
Trachea
ANATOMY
It is important to be conscious of the tracheal anatomy to have a better understanding of the pathophysiology of injuries caused during thyroidectomy.
The trachea is a tubular structure that connects the outside air with the lung’s parenchyma. It has an average length of 11.8 centimeters, starting at the cricoid cartilage and ending at the carina. During its course it is composed of 18 to 22 D-rings with an anterior C-shaped cartilage and a posterior membranous wall. The posterior aspect of the trachea is in close relationship with the esophagus, separated by the trachealis muscle.
There are three components of tracheal anatomy that thyroid surgeons should pay close attention to as these factors can play an important role during tracheal injuries. The first factor is the well-known relationship of the trachea with the thyroid gland. The thyroid isthmus crosses anterior to the trachea at the second to third tracheal ring. This close relationship makes the trachea prone to both direct injury during dissection and invasion by tumors of the thyroid that may require en bloc resection.
Together with the close proximity between the trachea and the thyroid comes a joint vascular supply. These two structures are supplied by the inferior thyroid artery. Injury to the vascular supply of the trachea can predispose to tracheal ischemia and with it, perforation. The tracheal branches arising at the proximal inferior thyroid artery approach the tracheal wall laterally and give superior and inferior branches that anastomose with the contralateral side. Given this vascular configuration, circumferential dissection of the trachea of more than 1 to 2 cm predisposes to ischemia.
The last factor of tracheal anatomy that is worth mentioning is its delicate wall; its thickness averages 3 mm. It is critical to understand these anatomic facts and relationships in order to operate on the thyroid gland safely and prevent unnecessary injury.
INCIDENCE AND RISKS FACTORS
The true incidence of tracheal injury during thyroidectomies is not well known. Gosnell et al. in a study including 11,917 thyroidectomies during a 45-year period at a single institution found seven cases, for an incidence of 0.06%. In a study from 2018, performed by Tartaglia et al., an extensive literature review found only 16 patients reported. It is postulated by some authors that although it is a rare complication, it is underreported.
As previously mentioned, the reporting of tracheal injuries comes from case reports and case series. Risk factors for its occurrence have been postulated. Factors attributable to the tracheal wall are: thyrotoxic goiter that causes long-term tracheal compression that over time may cause tracheomalacia ; female sex as the trachea wall is thinner; and multi-nodular goiter that will cause intense fibrosis making the dissection plane more difficult. Excessive retraction of the gland while dissecting the ligament of Berry can cause distortion of the trachea, exposing the posterolateral surface to injury. In fact, at times the surgeon can become confused regarding the anatomy when excessive traction and rotation are applied to the thyroid. The membranous portion of the trachea can be exposed and pointing up at the surgeon and can be easily penetrated if the surgeon is not aware.
Factors associated with altered tracheal blood supply are: improper or prolonged intubation; elevated cuff pressure impeding adequate blood supply; and intraoperative bleeding with increased use of electrosurgical devices that can cause thermal injury. Hematoma formation is thought to cause compression and diminished vascular supply. Superinfection of the hematoma or primary deep surgical site infection may also predispose to tracheal necrosis and perforation.
Another contributing factor reported is acute elevation of the intrathoracic pressure with a Valsalva maneuver. This is hypothesized to act at an already weakened tracheal wall leading to perforation. Some authors suggest that patients should abstain from strenuous activities for a few weeks postoperatively and to have adequate control of coughing and sneezing.
CLINICAL PRESENTATION
There are two main presentations of tracheal injury that have been reported: one is the incidental intraoperative finding and the other and more feared is the delayed presentation that can happen days to weeks after surgery. All patients with subcutaneous emphysema after thyroidectomy should be consider to have a tracheal rupture until proven otherwise.
Preoperative findings concerning for malignant tumor invasion into the trachea include cough, hoarseness, hemoptysis, and dyspnea. Fiberoptic laryngoscopy may occasionally demonstrate tracheal invasion, but usually a thin-cut computed tomography (CT) scan through the trachea is necessary to determine invasion. If invasion is still uncertain at the time of surgery, then tracheoscopy should be performed at the outset of the procedure. The author (Owen) finds the best technique to be placement of a laryngeal mask airway (LMA) followed by insertion of a fiberoptic scope through the LMA for examination of the larynx and tracheal wall. This is suitable for a tumor with minimal invasion and widely patent airway, whereas a tumor that has penetrated the trachea widely and is causing near airway obstruction should be handled with an awake tracheostomy below the tumor, or consideration of cardiopulmonary bypass should be entertained if a tracheostomy cannot be placed due to the extensive nature of the tumor.
Well-differentiated thyroid cancer rarely invades other tissues; however, when this occurs it is controversial if the surgical management should include the removal of the affected structures or if the tumor should be “shaved off” with the possibility of leaving microscopically positive margins. In general, shaving tumor off of the trachea is acceptable if no gross disease is left behind and the trachea is not penetrated. In case of en bloc resection of the trachea, primary reconstruction can be attempted if less than six rings are involved. In other cases, flap reconstruction or permanent tracheostomy may be necessary.
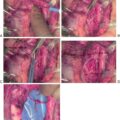
Removal of large tumors creates an increased risk for intraoperative damage. Intraoperative findings associated with tracheal injury are ongoing leak from the ventilatory system, bubbling at the surgical field, or even an exposed endotracheal tube. The most common site of injury is the posterolateral tracheal surface and, according to Gosnell et al., that is likely the result of excessive traction while dissecting the ligament of Berry. These injuries can be encountered during the dissection or might be recognized at the end of the procedure while doing a leak test. Given this possibility, it is recommended to perform a leak test at the end of the procedure by filling the surgical site with sterile saline and asking the anesthesiologist to perform a Valsalva maneuver.
A delayed presentation of tracheal injury can happen days or even weeks after the original surgery. This presentation is less common, but given its potential to compromise the airway, it can be lethal. These patients present with dyspnea, hoarseness, facial and neck swelling secondary to subcutaneous emphysema, and wound infection. A story of coughing or sneezing can be reported by the patient prior to the initiation of the symptoms. Once this lesion is encountered, one should be aware of potential concomitant complications including recurrent laryngeal nerve injury.
DIAGNOSIS AND INITIAL TREATMENT
Physicians taking care of these patients should have a high index of suspicion of a delayed presentation. Differential diagnosis includes esophageal injuries, pneumothorax, and necrotizing soft tissue infections. The priority should be to stabilize the patient following the airway, breathing, and circulation (ABC) principles of trauma. One should be prepared to perform an emergency tracheostomy or rapid-sequence endotracheal intubation using a fiberscope. In case of endotracheal intubation, the cuff should be positioned distal to the injury. This principle is also important in case of injuries found intraoperatively to maintain proper ventilation.
Once the patient is stabilized, including a secure airway, a chest X-ray should be done to rule out a pneumothorax. This will not reveal the tracheal injury, but secondary signs such as subcutaneous emphysema can be encountered. A CT scan of the chest and neck with IV contrast can demonstrate the defect. A normal CT scan does not rule out the diagnosis, as false negatives can be found in case of adjacent edema, secretions, or hemorrhage. Bronchoscopy (or tracheoscopy as described previously) can also be done to assess the injury. In case of concomitant sepsis, broad-spectrum antibiotics should be promptly started.
NONOPERATIVE MANAGEMENT
Tracheal injuries encountered intraoperatively should be repaired—surgical repair will be discussed in the following section. For patients with delayed presentation, once the diagnosis is made, the symptoms and most importantly the respiratory status will dictate further management. Even though most patients reported in the literature underwent surgical repair of the injury, a few cases of successful nonoperative management have been described.
Conzo et al. were the first to report the nonoperative management of a small tracheal perforation (1.5 mm) following thyroidectomy. Davies et al. followed with another case where a “pinhole”-size defect was managed expectantly with favorable results. Benson et al. also described in their case a pinhole-size defect (1 mm) found on bronchoscopy; his group managed the subcutaneous emphysema with a bedside exploration and a Penrose drain placement, which was removed after a few days. Gonzalez-Sanchez used intravenous antibiotics and bedside wound exploration, which caused a tracheal fistula that closed with conservative management. In all of these cases, the patients were mildly symptomatic from the subcutaneous emphysema, had no respiratory distress, and showed hemodynamic stability.
Mazeh et al. also attempted nonoperative management in their case. Given worsening symptoms 4 days after presentation, they pursued operative management where a 2.5-cm linear tear was found. These five cases suggest that it is reasonable to consider a trial of nonoperative treatment for stable patients with small defects (less than 1 cm).
Prior to discussion of the operative management of tracheal injuries during thyroidectomy, it is worth mentioning that some authors have tried a minimally invasive approach. One author proposed the use of covered stents into the trachea. This novel approach was reported by Han et al., where, at a single institution, two patients developed a tracheal perforation after thyroidectomy. In both cases the diagnosis was confirmed by bronchoscopy, and under fluoroscopic guidance, a 20- × 60-mm covered stent (Sewon Medical Co., Seoul, Korea) was placed. They had no recurrence of perforation at 31- and 35-month follow-up, respectively.
OPERATIVE MANAGEMENT
Urgent surgical management should be considered in those patients exhibiting cardiopulmonary instability, large pneumothorax, large pneumomediastinum, enlarging or symptomatic subcutaneous emphysema, and/or tracheal deviation. As mentioned previously, during the initial management the priority should be to secure an airway and treat based on the principles of trauma, treating any condition that interferes with cardiopulmonary stability (i.e., tamponade, tension pneumothorax).
Common operative findings include necrosis and larger defects, usually greater than 1 cm. Most of the lesions are in the posterolateral aspect of the trachea, but injuries to the anterior surface have also been reported. When necrosis is found, it is necessary to debride the trachea until healthy tissue is encountered. Once it is debrided, the injury size will determine the type of repair needed. Different approaches have been reported in the literature, including primary repair with absorbable sutures, primary repair reinforced with a fibrin-thrombin patch, primary repair with muscle flap reinforcement (strap muscles or sternocleidomastoid), circumferential tracheal excision with anastomosis, or tracheostomy placement. Postoperative drain placement after the repair is controversial: there is no evidence that one approach is better compared with another, and given the rarity of this entity, is in unlikely we will have comparative studies between techniques. ,
Concomitant infection can be found at the time of the surgical exploration. It is not clear if the infection predisposes to necrosis and perforation or the perforation causes secondary colonization and infection. Studies reporting associated infection have found Staphylococcus and Streptococcus species in the cultures. In those cases, broad-spectrum antibiotics should be promptly started and then therapy should be tailored based on cultures. One case also reported the successful treatment of a large infected tracheal defect with a vacuum-assisted closure therapy (VAC).
CONCLUSION
Tracheal injury after thyroidectomy is a rare complication. It can be encountered intraoperatively or weeks after the index operation. There are multiple surgical procedures for its management. A high index of suspicion is needed for those with a delayed presentation, and subcutaneous emphysema should prompt further investigation. Cardiopulmonary stability will dictate the treatment.
Esophagus
ANATOMY
As stated for tracheal injuries, knowledge of esophageal anatomy is crucial and will help understand the mechanism of an injury caused during thyroidectomy.
The esophagus is a muscular tube-like organ that starts at the pharynx at the level of the sixth cervical vertebra. During its course it crosses three anatomical compartments: the neck, the thorax, and the abdomen. Through its course it has relationships with different structures. In the neck, the esophagus lies posterior to the larynx and trachea just left of the midline. The posterior border of the cervical esophagus is marked by the prevertebral cervical fascia. Although the esophagus is not always in direct contact with the thyroid, rarely, large or invasive tumors can sometimes penetrate the esophagus.
Different from other gastrointestinal organs, the esophagus lacks a serosa. Its architecture consists of three layers: mucosa, submucosa, and muscularis. Important anatomical considerations for esophageal repair are that the submucosa is the strongest layer and that the absence of serosa can make repair a challenge. In regard to the muscular layer, the cervical esophagus is composed of skeletal muscle, whereas the lower esophagus is made up of smooth muscle.
Given the long course of the esophagus through three different anatomical regions, the blood supply comes from various branches at each level. The cervical esophagus receives its supply from the inferior thyroid artery. The thoracic and abdominal esophagus are supplied by branches of the thoracic aorta and the left gastric artery, respectively. Just as described in tracheal injuries, proximal ligation of the inferior thyroid artery can predispose to ischemia and perforation. Venous drainage at the neck follows the arterial supply via the inferior thyroid vein; however, the esophagus has an extensive submucosal venous plexus.
INCIDENCE AND RISK FACTORS
Esophageal injury during thyroidectomy is even more rare than tracheal injuries with only a few reported cases in the literature. , No studies have reported the actual incidence of this complication. The incidence of esophageal perforation not associated with thyroidectomy is also low, with only 3.1/1,000,000 per year. Based on the study by Gomez-Ramirez, esophageal perforation during thyroidectomy occurs less than 0.0001% of the time. However, the mortality reported for esophageal perforations can be as high as 29%.
Risks factors identified in the available case reports are reoperation, difficult intubation, and the use of laryngeal mask. , Esophageal perforations occurring during difficult intubation or nasogastric tube placement can occur even in nonthyroid surgery. It is well known to surgeons the added difficulty of operating in a previously operated field; in cases where a completion thyroidectomy is needed, the surgeon should be extra meticulous, as the normal tissue planes will be disturbed with excessive fibrosis and scar tissue.
One particular circumstance that is important to discuss is that of a patient with anaplastic thyroid cancer. Patients generally present with a rapidly growing irregular mass of the thyroid, often with symptoms of airway compromise and dysphagia. Fine-needle aspiration biopsy is useful to suggest the diagnosis, but definitive diagnosis with core needle biopsy or open biopsy is generally required. Whereas for other varieties of thyroid cancer surgical extirpation is the mainstay of treatment and the best hope for cure, in anaplastic thyroid cancer this is not the case. Rather, aggressive surgery can result in tracheal or esophageal perforation. Esophageal perforation in particular will generally never close in this setting. Thus, a patient with a terrible disease and very limited life span will now have the additional morbidity of an esophageal fistula. This may also prevent the administration of radiation and/or chemotherapy or participation in a clinical trial. Thus, if anaplastic thyroid cancer is suspected, a biopsy should be taken and the incision closed until the result is known and a well-informed plan can be formulated.
CLINICAL PRESENTATION
In patients who will undergo thyroidectomy who complain of dysphagia, a suspicion of esophageal invasion should be raised. Direct invasion into the esophagus by differentiated thyroid carcinoma ranges from 1% to 3.2%. In cases where there is preoperative concern, diagnostic testing should include esophagoscopy with or without endoscopic ultrasound to exclude esophageal involvement prior to undergoing a thyroidectomy.
Esophageal injuries can be recognized intraoperatively by the presence of exposed esophageal mucosa or thick secretions caused by saliva. Excessive traction of the thyroid may lead to esophageal injury. In cases of uncertain location of the esophagus or where its relationship with the thyroid is unsure, a nasogastric tube can be placed by the anesthesiologist to help palpate and better localize its course. Intraoperative esophagoscopy can also be considered in cases where there is concern for injury.
Postoperatively, patients can present with wound discharge, wound infection, or subcutaneous emphysema. In some cases where a drain is left in place, the output will be noted to be turbid, raising the concern of esophageal or lymphatic leak. Other symptoms reported by patients can be vomiting, dysphagia, and dyspnea. Patients can also present with florid sepsis; however, cervical esophageal perforations are much better tolerated, compared with perforations in the thorax and the abdomen. Cervical esophageal perforation can progress to mediastinitis, cervical abscess, and carotid pseudoaneurysm if it is not promptly recognized and treated.
DIAGNOSIS AND INITIAL TREATMENT
A delay in treatment has a direct correlation with mortality in esophageal injuries. The natural history of the disease progresses from contamination of the surrounding spaces to sepsis and death in as quick as 24 to 48 hours. As the mortality of this injury is related to sepsis, resuscitation with crystalloids and early administration of intravenous broad-spectrum antibiotics is key during the initial phase. The patient has to be nothing by mouth (NPO). In association with resuscitation, it is important to obtain source control either with surgical or minimally invasive techniques.
Antibiotic coverage should be targeted toward both aerobic and anaerobic bacteria. Antifungal therapy is controversial. The Infectious Diseases Society of America does not recommend antifungal coverage for community-acquired gastrointestinal perforations, but there is a role for antifungals in health care–associated infections and immunocompromised patients. In a series of 27 patients with esophageal perforation, Elsayed et al. found that patients with positive fungal cultures had the worst outcomes, including increased mortality, hospital stay, and intensive care unit (ICU) stay.
Esophageal perforation can be seen in different imaging studies. Chest and neck X-ray will only show nonspecific findings such as subcutaneous emphysema, pleural effusion, pneumomediastinum, and pneumothorax. Contrast-enhanced CT is proposed by the World Society of Emergent Surgery Guidelines as the test of choice with a sensitivity of 92% to 100%. Esophagogram with gastrografin or barium can also provide valuable information, especially whether the perforation is contained or not. Endoscopy can be used as a diagnostic tool in cases where imaging is inconclusive.
NONOPERATIVE MANAGEMENT
Once the diagnosis is made and the patient has been stabilized, the surgeon has to decide if the patient warrants a reoperation or if the injury can be managed nonoperatively. The risks and benefits of both possibilities have to be compared; reoperation can be challenging, as the planes could be disrupted given the inflammation, increasing the risk of further damage. However, if the patient has sepsis or worsening symptoms, surgery is needed.
Nonoperative treatment can be offered to those patients with an early presentation, a contained perforation, and who show no evidence of systemic symptoms or cardiovascular instability. These patients should be under close surveillance (i.e., ICU). , Surgical management needs to be considered if these criteria are absent at presentation or during follow-up. One way of making this decision is using the Pittsburgh esophageal perforation severity scoring system. This calculator uses 10 clinical and radiological variables to classify patients into low-, medium-, and high-risk groups. Only low-risk group patients are potential candidates for nonoperative management.
Nonoperative management is similar to the initial treatment of esophageal perforations. It includes having patients NPO, coverage with broad-spectrum antibiotics, and appropriate control of the leak with a drain. If a drain has not been placed during surgery, then a drain should be placed into the area of concern. In addition to these elements, a cornerstone of this management is adequate nutritional support. Nutrition can be given via different routes including total parenteral nutrition, or tube feeds via nasogastric tube or gastrostomy tube. (However, a nasogastric tube can be placed under direct vision at surgery, but should not be attempted blindly in a patient with an esophageal injury.) Proton pump inhibitors should be started as well.
Two of the case reports of esophageal injuries caused by thyroidectomy have described successful nonoperative management. , In both cases, suction drains were left during the index operation, and turbid discharge was noted postoperatively. The presence of drains provided control of the fistula, avoiding the need for surgical reexploration. In most thyroidectomies the placement of a drain is not routine, but it should be considered if there is concern for an esophageal injury. With control of the fistula, nonsurgical management in selected cases with broad-spectrum antibiotics and nutrition supplementation is an option. Once the drainage decreases and an esophagogram shows no fistula, the drains can be removed.
ENDOSCOPIC MANAGEMENT
As previously mentioned, esophageal perforations can be evaluated with endoscopy when the diagnosis by CT is uncertain. Endoscopic evaluation should be performed with caution, as it can potentially aggravate the injury. Once the injury has been identified, there are therapeutic options that have been described in case series and retrospective studies. The three endoscopic therapeutic options are: endoclips, covered stents, and endoscopic vacuum therapy.
Endoclips come in two different types: through the scope and over the scope. The former are considered for smaller perforations, usually less than 10 mm, whereas the latter can cover areas up to 30 mm. The overall reported success rate for endoscopic clips is between 45% and 95%. Factors implicated in their success include anatomical location, chronicity, type of injury (perforation vs leak vs fistula), and if they are used as primary or as rescue therapy. The best results are achieved with acute perforations where clips are used as the primary intervention.
Covered stents bypass the injury site, allowing it to heal and preventing ongoing contamination of the extraluminal spaces from luminal contents. There is a potential risk of stent migration in 10% to 37% of the cases that will require another procedure for its retrieval. Similar to the experience with endoscopic clips, the results are variable. Factors associated with failure include injuries greater than 6 cm and injuries of the cervical esophagus given that stents at this location are not generally well tolerated. Ozer et al. reported the successful use of a covered stent in combination with surgical exploration and muscle reconstruction in a case of esophageal injury during thyroidectomy. Their patient had no complications or complaints from the stent. However, patients with proximal esophageal stents sometimes report pain and discomfort.
It is worth mentioning the endoscopic vacuum therapy for esophageal perforations. This relatively new technique that was first described in 2006 works similarly to a traditional vacuum device promoting granulation tissue. The sponge is introduced endoscopically into the esophageal perforation or abscess cavity. Möschler et al. reported the successful treatment of two cases with proximal iatrogenic esophageal injuries using endoscopic vacuum therapy.
OPERATIVE MANAGEMENT
Operative management is usually offered to those patients who present with sepsis or have deterioration while undergoing a nonoperative approach. At surgery, definitive repair is preferable but will not always be feasible, and a judgment may be made to minimize damage and drain widely instead. Each case will be different, and treatment should be tailored to the patient based on the presentation, surgeon experience, and comfort.
In a case of thyroidectomy for a T2 papillary thyroid cancer, Conzo et al. encountered a fistula at the pharyngo-esophageal junction on a Gastrografin swallow. The patient exhibited signs of wound infection without systemic signs; they took their patient back for reexploration and drain placement without esophageal repair. The presence of the drain permitted control of the fistula, which healed 15 days later with proper nutritional support and antibiotics. The size and location of the defect are not mentioned.
In cases where an intraoperative esophageal defect is noted either from iatrogenic injury or due to a concomitant resection given tumor invasion, treatment will differ depending on the depth of the injury. Primary repair can be done where the injury is only muscular without affecting the mucosa or submucosa. Full-thickness injuries require muscular flap repair, gastric pull-up, or jejunal free flap repair for circumferential defects. For cases where primary repair is done, surgeons should be careful not to suture the “backwall” of the esophagus. Peng et al. described a case of esophageal stenosis in a case where an esophageal injury during thyroidectomy was primarily repaired.
Multiple procedures have been described to repair cervical esophageal injuries. Some of the possible options include repair over T-tubes, repair with muscle flaps, resection and reconstruction with gastric pull-up, jejunal free flaps, or colon transposition. Esophageal resection is a major operation. The reader should be conscious that these complex reconstruction techniques are not free of risk and should only be attempted by those with proper experience.
More aggressive interventions to achieve control of the fistula include exclusion and diversion with an esophagostomy. Cervical esophagostomy has been in the armamentarium of the surgeon for a long time but is infrequently used. This intervention with a high mortality (15% to 40%) could appear to be radical after a thyroidectomy; however, complete diversion can be lifesaving in cases with ongoing contamination where other techniques have failed. As we have mentioned before, control of sepsis is crucial, and oral secretions have a high concentration of bacteria that can be exteriorized with complete diversion.
The cervical esophagus is better approached via a left incision anterior and parallel to the sternocleidomastoid. The carotid sheet is retracted laterally, and once the esophagus is identified, a Penrose is placed around it to help retract it to the incision. While encircling the esophagus, care should be taken not to injure the recurrent laryngeal nerve. The esophagus is then dissected for mobilization superiorly and inferiorly. Once it is free, it is transected and ligated distally, and the proximal end is exteriorized and matured. , One of the major disadvantages of this procedure is the need for further and more complex reconstruction.
CONCLUSION
Esophageal injuries are rare and even more rare after thyroidectomies. Despite its low frequency, the injury carries a high rate of mortality. Early treatment includes broad-spectrum antibiotics and source control. Nonoperative management can be considered for stable patients, whereas those with instability should be emergently explored. Surgeons operating on the thyroid should be aware of this complication, including its management.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree