Fig. 8.1
Typical timeline of mammographic (MG) findings following Breast Conservation Therapy
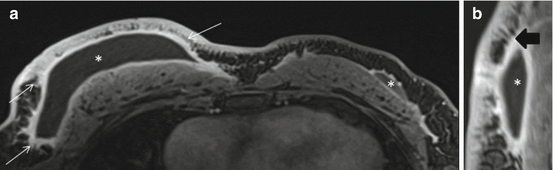
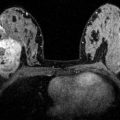
Early findings of postoperative collections such as seroma and/or hematoma, breast edema and skin thickening, are most pronounced within the first 6 months (Fig. 8.1). Postoperative seromas/hematomas are relatively discrete hyperdense oval masses on mammogram, occasionally with internal dependent layering, confirming it as a fluid collection (Fig. 8.2). Ultrasound helps further characterize postoperative collections if findings on mammogram are unclear, typically showing a simple or complex fluid collection closely associated with the surgical incision seen as a hypoechoic track extending to the skin (Figs. 8.3 and 8.4). On MRI, a seroma appears as a circumscribed fluid-filled structure with smooth rim enhancement (Fig. 8.5), and a hematoma appears as a nonenhancing mass of varying internal signal depending on chronicity. Although seromas can have benign nodular or irregular enhancing components due to granulation tissue or fat necrosis, these should be viewed with suspicion and residual/recurrent malignancy must be excluded (Fig. 8.6). Postoperative collections usually resolve by one year, but some can persist indefinitely. As a collection decreases in size, the diminishing surgical cavity evolves into a coalescing scar made of dense connective tissue and fibrosis. This is seen on mammogram as an area of architectural distortion with central equal/low density and peripheral radiating spiculations interspersed by fat. This classic appearance of fibrotic bands of a scar entrapping areas of fat necrosis is well depicted on digital breast tomosynthesis (Fig. 8.7a, b). In contrast, a cancer associated with spiculations due to adjacent desmoplastic reactions is more hyperdense and mass-like centrally on mammogram. Benign fat necrosis calcifications at lumpectomy scar typically develop 2–5 years post radiation, and early evolving benign calcifications may mimic malignancy. On ultrasound, scarring typically appears as hypoechoic spiculations extending to the skin (Fig. 8.7c). On MRI, post lumpectomy scar tissue may enhance for up to 18 months, but after this period enhancement is expected to subside [7] (Fig. 8.8). New or increased enhancement at the scar on MRI, particularly after 18 months, requires exclusion of recurrent disease. However, in our practice on our 3.0T magnet, we have seen enhancement at the lumpectomy site several years after surgery. In these cases, we verify that the enhancement is not increasing as compared with prior exams. This enhancement may be explained by the relatively increased relaxation time for both fat and glandular tissue at 3T compared to that of gadolinium. The resulting relative difference in signal intensity between enhancing lesions and nonenhancing tissues is therefore increased at 3T, thus making enhancing lesions more conspicuous [8, 9].
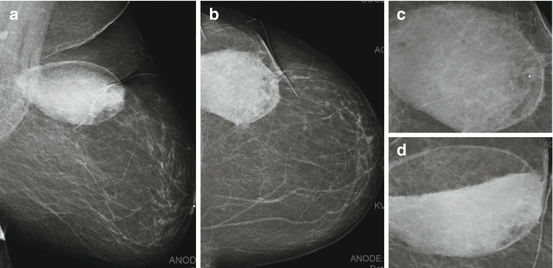
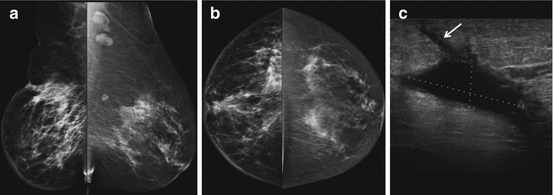
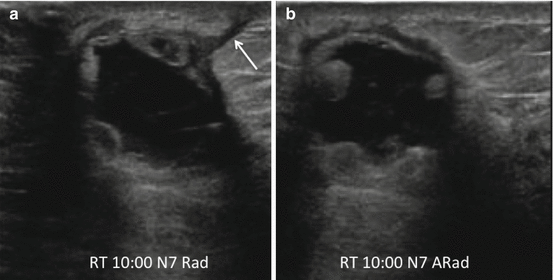
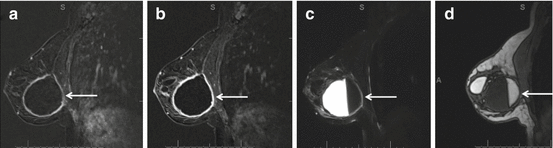
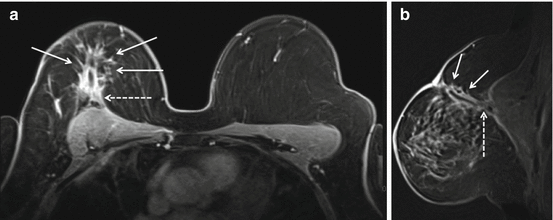
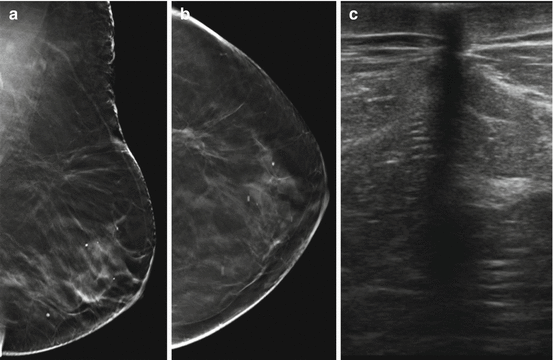
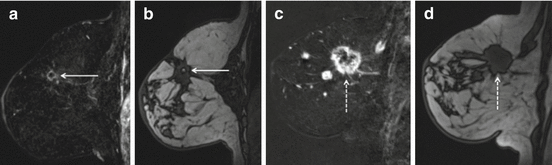

Fig. 8.2
Status post recent left lumpectomy with a circumscribed hyperdense oval mass in the surgical bed in the upper outer quadrant deep to the scar marker on MLO (a) and CC views (b). Delayed lateral view (d) demonstrates layering of internal fluid, which appears dense en face (c), consistent with a post surgical fluid collection

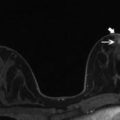
Fig. 8.3
Four months following right lumpectomy and radiation therapy for breast cancer. There is moderate skin thickening and breast edema (trabecular thickening) involving the right breast seen on MLO view (a) and CC view (b) as compared to the untreated normal appearing left breast. There is an anechoic postoperative seroma in the superior right breast in the surgical bed, extending to the skin at the site of surgical excision (arrow) (c)

Fig. 8.4
Five months following right lumpectomy and radiation therapy, there is a thick walled complex fluid collection with suggestion of internal septations (a, b) in the surgical bed with peripheral nodular granulation tissue and scarring extending to skin (a) (arrow) at the level of the surgical incision

Fig. 8.5
Post surgical seroma with circumscribed margin and smooth thin rim persistent enhancement on T1 post contrast subtraction images at early (a) and late (b) phases. Internal fat-fluid level (c, d) is related to history of prior free fat injection in this patient

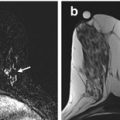
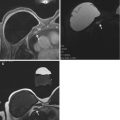
Fig. 8.6
Fifteen months status post right breast lumpectomy and radiation therapy. Axial (a) and sagittal (b) T1 post contrast images show a nearly completely resolved seroma surrounded by a thickened and nodular residual capsule (a) and new heterogeneous irregular spiculated peripheral enhancement (b) (arrows, solid). In addition, the posterior aspect of the irregular enhancement demonstrates washout kinetics on imaging and correlates with positive deep surgical margin at histologic analysis (a, b) (arrows, dashed). This underwent MR guided biopsy, confirming presence of residual DCIS

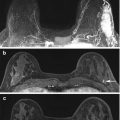
Fig. 8.7
Left lumpectomy scar seen on tomosynthesis images on MLO (a) and CC (b) views, showing central lucent fatty attenuation and peripheral spiculations of fibrotic bands interspersed with fat. A separate case of post lumpectomy scar seen on ultrasound as a hypoechoic band extending to the skin (c)

Fig. 8.8
Post lumpectomy breast on T1 post contrast subtraction (a) and T1 non fat saturated (b) sequences show an area of retracted scarring containing central area of fat with rim enhancement, consistent with fat necrosis. This is in stark comparison with T1 post contrast subtraction (c) and T1 non fat saturated (d) sequences showing the same breast with interval increased bulk and now mass like configuration of the scar, with tumor replacement of previously seen central fat, consistent with recurrent malignancy. In addition to the new irregular enhancing mass at the scar, there is additional adjacent satellite disease (c)
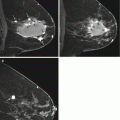
Following whole breast radiation, skin thickening and breast edema share similar timelines of recovery to near normal/normal by approximately 2 years (Fig. 8.1). Skin thickening and breast edema (trabecular thickening) are best evaluated in comparison with the contralateral normal breast on mammogram (Fig. 8.3a, b), ultrasound, and MRI (Fig. 8.9). On MRI, post radiation “quiescence” in the treated breast may also be seen as asymmetrically decreased background parenchymal enhancement and fibrocystic changes (Fig. 8.10). Overall, new or increased findings usually warrant biopsy to exclude recurrent disease given expected pattern of evolution/resolution of most post surgical changes over time.
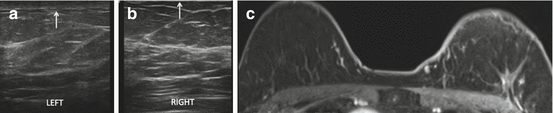
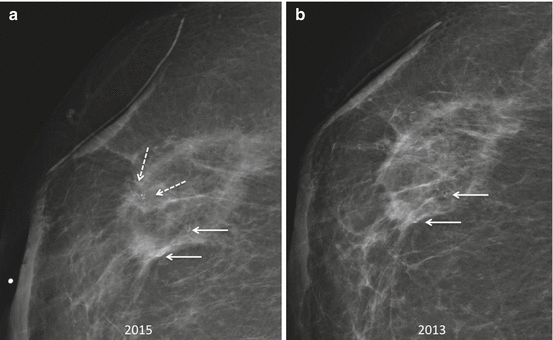

Fig. 8.9
Five months following left lumpectomy and radiation therapy for breast cancer. Asymmetric left breast skin thickening (arrow) (a) and tissue edema which manifests as diffusely increased stromal echogenicity (a) are seen as compared to the normal right breast (b). Similarly, left breast skin thickening is seen on MRI (c)

Fig. 8.10
New pleomorphic calcifications in the right lumpectomy bed two and half years following breast conservation therapy for invasive ductal carcinoma (a) (arrows, dashed) were biopsied yielding DCIS, consistent with recurrence. In contrast, the adjacent curvilinear slow evolving calcifications at the periphery of an area of fat necrosis are distinct in appearance (a, b) (arrows, solid). Nevertheless, there is significant overlap in appearance between early evolving calcifications of fat necrosis and malignant calcifications
8.2 Tumor Recurrence and Imaging Surveillance
True recurrence occurs at the site of original tumor and signifies local treatment failure. Recurrence is rare before 2 years post treatment, but may occur as early as 2–5 years following BCT [6]. Patients who undergo lumpectomy without radiation, have positive surgical margins, multifocal disease, or ER-negative cancers are at further increased risk of recurrence [10]. Up to 50 % of recurrence is detected on mammogram, which can present as new suspicious calcifications in the lumpectomy bed (Fig. 8.10), developing asymmetry, new mass or architectual distortion. Ultrasound may help to identify a mass at or adjacent to the scar. MRI is useful in distinguishing between benign entities such as a scar or fat necrosis from recurrence when mammogram and ultrasound are indeterminate. Although protocols vary, most centers perform post treatment mammographic surveillance every 6 months following BCT for 2–5 years with subsequent annual mammograms. This is accompanied by annual clinical breast exam to detect imaging occult recurrence.
8.3 Mastectomy
Conventional indications for mastectomy were covered earlier in this chapter as were contraindications for BCT. In addition, some patients eligible for BCT choose mastectomy instead. Contralateral prophylactic mastectomy is increasingly requested in the setting of unilateral breast cancer and bilateral prophylactic mastectomy is accepted treatment for high risk patients, typically in the setting of BRCA mutations [11].
8.3.1 Techniques
Modified radical mastectomy (MRM) is, in the absence of pectoralis muscle invasion, the most extensive mastectomy performed today and includes complete removal of the breast tissue, skin envelope, nipple areolar complex and level I and II axillary nodes. This surgery is appropriate in locally advanced cancers including inflammatory breast cancers and is the surgery of choice when a woman is not planning reconstruction [12]. Newer techniques include the skin sparing mastectomy (SSM), which preserves the skin envelope and inframammary fold while removing the breast tissue and nipple areolar complex [13], and the nipple sparing mastectomy (NSM), which preserves the skin envelope and nipple areolar complex while removing the breast tissue. SSM facilitates breast reconstruction, provides a superior cosmetic outcome as compared with MRM, and can often be performed in lieu of MRM, provided there is approximately 5 mm between the tumor margin and the skin. NSM provides the best cosmetic outcome and is most often performed in the prophylactic setting. In the setting of cancer treatment, indications for NSM put forth by Voltura et al. include a tumor size <4.5 cm, distance from the areola of >2.5 cm and distance from the center of the nipple >4 cm [14].
8.3.2 Imaging Findings
The post-mastectomy or post-reconstructed breast is not usually imaged to screen for recurrent disease. However, MRI has been shown to be useful in evaluating the post-mastectomy or post-resonstruction breast for post-treatment changes versus recurrence. In addition, imaging of mastectomy or reconstruction may occur during MRI evaluation of the contralateral breast. In MRM without reconstruction, MR imaging findings are straightforward and include the absence of a breast with simply the pectoralis muscles, subcutaneous fat and overlying skin. As in any post-operative setting, edema, presenting as increased skin thickness and increased signal on T2 weighted images will be present initially and will subside over time. As in any post-operative setting, seromas and hematomas can form. Clean seromas can be distinguished by smooth, thin margins. However, seromas may have shaggier enhancing margins which can raise concern for tumor (Fig. 8.11). In this case, lesion enhancement kinetics and pathology reports can be useful. Progressive enhancement is more consistent with post-operative changes and scarring while washout enhancement is more concerning for malignancy. With reported clean margins, when imaging is within 6 months of the date of surgery, and when the enhancement pattern is persistent, a short-interval (6 month) follow-up study may be appropriate in lieu of biopsy. However, in cases without clean margins or in any case where enhancement demonstrates wash-out kinetics or has increased compared with a prior study, biopsy should be performed.