Fig. 9.1
Bilateral retroperitoneal metastasis of left testicular tumor, medializing aorta and vena cava
Post-chemo residual masses are teratomas in 40% of NSGCT cases [11]. In a study including 210 teratomas after PC-RPLND, the rate of mature teratomas , immature teratomas, and malignant transformation was 85, 7, and 8%, respectively [12]. Besides its chemo-resistant nature, teratomas are associated with debilitating complications such as “growing teratoma syndrome” and malignant transformation. These findings and associated risks enlighten the importance of surgery [13–15].
Should we do PC-RPLND for all the patients? Computed tomography (CT) or magnetic resonance imaging (MRI) findings are inadequate to determine the presence of a viable tumor or teratoma [16, 17]. Kollmannsberger et al. evaluated residual mass viability in 85 masses in 45 patients and reported that FDG-PET scans had 59% sensitivity and 92% specificity [18]. A prospective study evaluated 60 residual tumors in 28 GCT patients after high-dose chemotherapy and concluded that PET seems useful in patients with stable disease or partial remission in CT/MRI and normal or marker-negative disease [19]. In another multicenter prospective study of 121 patients with stage IIC or III NSGCT, it was reported that sensitivity and specificity of FDG-PET were 70 and 48, respectively [20]. Prediction of tumor viability with FDG-PET was correct in 56% and was not better than the accuracy of CT (55%) or markers.
Various models were constructed to better decide usefulness of either immediate resection of a residual mass or follow-up. Steyerberg et al. developed a statistical model predicting the histology necrosis, mature teratoma, or cancer after chemotherapy. Predictors of necrosis were defined as the absence of teratoma elements in the primary tumor, pre-chemo normal alpha-fetoprotein (AFP) , normal human chorionic gonadotropin (HCG) and elevated lactate dehydrogenase (LDH) levels, small pre- or post-chemotherapy mass, and large shrinkage of the mass during chemotherapy [21]. This model was updated in 2007 to select patients for surgery, particularly for patients with small residual masses and low predicted probabilities of benign tissue [22].
In NSGCTs, PET scan has no superiority over CT or tumor markers. Besides, the designed models are inadequate to predict histology. Of 276 post-chemo NSGCT patients, Kollmannsberger et al. reported that 161 achieved radiographic complete remission (CR) defined as minimal residual tissue ≤1 cm [23]. Eight of the ten relapses in the CR group were treated surgically for teratoma alone, whereas two required salvage chemotherapy. Disease-specific survival for the CR group was 100% after a median follow-up of 52 months. In another retrospective study, 141 NSGCT patients who achieved a CR to first-line chemotherapy were observed without further therapy [24]. Relapse was detected in 12 patients (9%) after a median 15.5-year follow-up. Six relapses were in the retroperitoneum. Of these 12 patients, only four died of the disease. The estimated 15-year recurrence-free survival (RFS) and cancer-specific survival rates were 90 and 97%, respectively. Toner et al. reported results of ≤1.5 cm residual retroperitoneal masses in 39 patients. They had only three residual viable tumors and five teratomas resected [16]. They’ve recommended RPLND for all patients with initial bulky retroperitoneal metastases (≥3 cm in diameter) irrespective of post-chemotherapy CT findings. On the contrary, Carver et al. analyzed the results of 532 post-chemo RPLND cases and reported 40% teratoma rate and 11% of them had post-chemo residual mass smaller than 1 cm [11].
For PC-RPLND, marker normalization is required first. The marker that is elevated after chemotherapy is directly related to the presence of viable tumor. However, the expected time for marker normalization may prolong in patients with very high HCG levels before chemotherapy. Marker decrease is expected in the presence of plateau marker after post-chemotherapy. Cystic teratoma may also cause elevated but stable marker level. In the presence of plateau but not rising marker levels after chemotherapy, RPLND is a treatment option [25].
Post-chemo residual tumor resection is performed for viable tumor or teratoma histologies in NSGCT. Those are detected in about 50% of stage IIC and higher stages. Today, there is no imaging or laboratory method predicting final pathology with a high accuracy. As a conclusion, post-chemo surgery is recommended to all residual masses larger than 1 cm [20, 26–28]. Another important data shows us that there is 6–9% relapse rate in masses smaller than 1 cm when observed [23, 24].
9.3 PC-RPLND for Advanced Seminoma
Although the risk of teratoma and malign transformation in seminomas is less worrying and in case of viable tumor the surgery is less effective on outcomes, PC-RPLND is still an important treatment option in seminoma treatment [29]. Similarly, post-chemo RT has no place in seminoma.
The tumor size in post-chemo seminoma has prognostic importance. Flechon et al. reported 13% of 79 patients had viable tumor on final pathology and all had residual masses larger than 3 cm in preoperative imaging [30]. Similarly Hofmockel et al. found one viable seminoma case in ten PC-RPLND patients whose residual mass was 5 cm in preoperative imaging [31]. Herr et al. studied 55 seminoma patients and noticed that only 30% (8/27) of patients with 3 cm or larger residual mass on CT had viable tumor (six seminomas and two teratomas) [32]. No viable tumor was detected in masses smaller than 3 cm. Although PC-RPLND lowers recurrence in cases with viable tumor, there is similar recurrence rate between both viable tumor and necrosis pathology. Puc et al. assessed 104 advanced seminoma patients who had achieved a complete response or partial response to induction cisplatin-based chemotherapy [33]. Their results on RPLND were assessed and correlated with pre-chemotherapy and post-chemotherapy characteristics. They found that two of 74 patients (3%) with residual masses less than 3 cm were considered site failures (had viable tumor on RPLND), compared with eight of 30 (27%) with residual masses > or = 3 cm.
Ganjoo et al. studied 29 advanced seminoma patients with post-chemotherapy residual masses and concluded that PET scans have no apparent benefit in PC evaluation of residual masses in bulky seminoma [34]. Another study analyzed the results of 37 seminoma patients and reported the cutoff point for PET scan to be 3 cm [35]. De Santis et al. analyzed the results of 56 patients with pure seminoma with post-chemo residual masses [36]. Only two patients with residual mass less than 3 cm had false-negative results. Positive predictive value was 100%. PET scans should be scheduled 6–8 weeks after chemotherapy. Three cm is the accepted cutoff size for PET scan. 2-18fluoro-deoxy-D-glucose FDG PET is the best predictor of viable residual tumor in post-chemotherapy seminoma residuals.
9.4 RPLND Borders
In the middle of the twentieth century, RPLND started having superiority over orchiectomy and RT [37]. In the 1960s, with the use of lymphangiography, it was shown that testis lymphatics drain to suprahilar LNs, and right testis lymphatics may drain to the left and vice versa [38]. Initially, extended dissection bypassed suprahilar LNs and included paracaval, precaval, para-aortic, preaortic, interaortacaval, and common iliac regions. Ray and Whitmore retrospectively analyzed the location of retroperitoneal lymph node metastasis of 283 patients who underwent RPLND [39]. They found that lymphatic metastasis was to the left side of para-aortic area in case of left-sided tumor and to variable locations in case of right-sided tumor. Donohue et al., in their study of 104 stage II patients, demonstrated that lymphatic metastasis from right-sided tumor was to interaortacaval nodes followed by precaval and preaortic nodes while in case of left-sided tumors was to para-aortic and preaortic areas followed by interaortacaval [40]. Weissbach and Boedefeld evaluated 214 consecutive patients with stage II disease (excluding bulky disease) with respect to localization relative to the side of the relative testis [41]. Solitary nodes of the right-sided and left-sided tumors were primarily located in the interaortacaval and para-aortic areas, respectively. Testicular Tumor Study Group suggests specific template RPLND which includes paracaval, precaval, interaortacaval, preaortic, and ipsilateral ileac dissection for right-sided testis tumors and preaortic and para-aortic LND for left-sided tumors. These modified templates catch 95% of metastatic LNs. As these studies lack long-term results, it’s hard to determine the true localization and ratio of recurrence. Here in the figure, you can see para-aortic and left parailiac metastasis of right testicular tumor (Fig. 9.2).
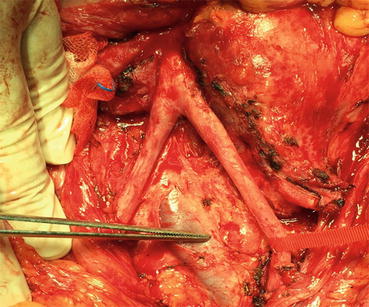

Fig. 9.2
Left parailiac and para-aortic metastasis of right testicular tumor
The sympathetic nerves originate from thoracic and upper lumbar areas synapse with paravertebral and hypogastric ganglions. Then, they spread to the pelvic organs along with the epigastric and presacral fibers. That’s why ejaculation and fertility could be affected negatively by RPLND. In the early 1980s, Narayan et al. reported that 50% of patients had normal spontaneous ejaculation postoperatively [42]. This considered the start point for RPLND. In the same decade, Donohue et al. studied 75 patients, most of whom had stage I, and found that most of them had postoperative normal antegrade ejaculation [43]. Heidenreich et al. published the results of 152 PC-RPLND with median follow-up of 39 months [44]. Modified template RPLND was done to 98 patients with a mass less or equal to 5 cm in the primary landing zone. Radical template RPLND was done to the other 54 patients. Antegrade ejaculation was preserved in 85 and 25% of patients undergoing modified and bilateral PC-RPLND, respectively. Only one of the eight recurrences documented was in the modified group. Although short follow-up period and radical type being done to large masses limit these results, in well-defined masses, a modified template PC-RPLND does not interfere with oncologic outcome but decreases treatment-associated morbidity [45]. The main mechanism by which modified template RPLND prevents nerve injury is the limited dissection on the other side. The surgeon’s effort to identify, dissect, and preserve sympathetic nerves is also essential. By this way, nerve-sparing intervention is a choice even for patients who undergo full template RPLND. Researchers suggested electrostimulation for intraoperative sympathetic nerve identification [46]. One should never forget about oncological principles while preserving nerves in testis cancer surgery.
9.5 Limitations of Modified Template RPLND
The most important argument for the use of modified templates is “the lower risk of other side lymphatic metastases, easier surgery when limited dissection, and lower complication rates.” Most of these techniques were initially defined for stage I disease. Later, especially with the advance in effective chemotherapy, they’ve preferred for advanced stages too. The absence of long-term follow-up periods in mapping studies considered a major limitation for these techniques [39, 40]. If resected specimens are sent to pathology laboratories in different bags, it would be helpful for pathologists to correctly stage extra-template disease (Fig. 9.3).
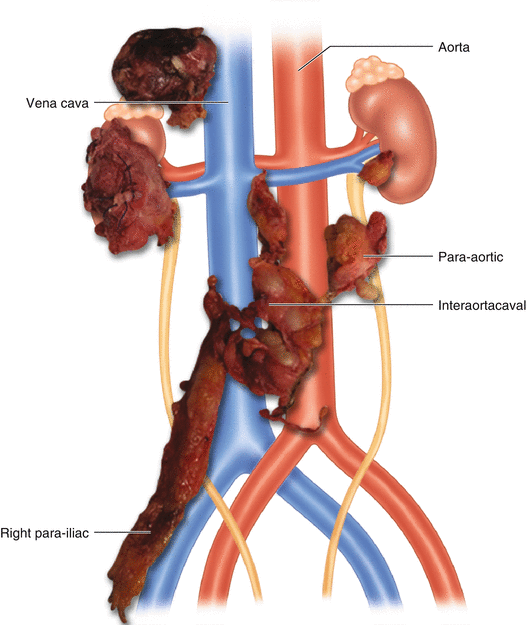

Fig. 9.3
Resected specimens sent to the pathology in different bags
Carver et al. studied 532 PC-RPLND cases [47]. Of all, 269 had either viable tumor or teratoma and 7–32% had extra-template disease. The most common extra-template area was interaortacaval and paracaval location for left-sided tumors and preaortic and para-aortic region for right-sided tumors. Residual mass size was reported to be the most significant indicator for extra-template disease. For residual masses larger than 5 cm, the incidence was as high as 25%. Another study including 500 cases has shown 1–11% and 5–33% risk of extra-template disease for pN1 and pN2/3 stages, respectively [48]. Extra-template disease risk for 191 pathological stage II patients was reported to be 3–23%. The incidence was decreased to 2–3% by inclusion of para-aortic, preaortic, and right common iliac regions to right-side templates and by inclusion of interaortacaval, precaval, paracaval, and left common iliac regions to left-side templates.
Another important issue is the impact of size and number of excised lymph nodes on survival. Carver et al. found that increasing post-chemo nodal size and decreasing lymph node counts were significant predictors of recurrence in 628 PC-RPLND cases. The 2-year RFS rates for >10, >30, and >50 lymph nodes were 90, 95, and 97%, respectively [49].
9.6 Open PC-RPLND
Thoracoabdominal approach is a choice when easy access is required to reach suprahilar lymphatics. On the other hand, transabdominal approach is fast and relatively easy to do and offers good accessibility to both sides. In the last years, midline extraperitoneal approach has been reported in small number of patients. Donohue defined RPLND to be a vascular surgery [50]. After opening anterior abdominal wall and peritoneum, using a retractor (Bookwalter) may help getting a good look. After that, by opening the posterior peritoneum from inferior to IMA area, retroperitoneum anatomy will be revealed. Dissection in the defined borders is usually done by lymphatic ligation or clipping. For large residual masses or in case of surgical difficulties, IMA could be sacrificed in order to expose para-aortic and left renal hilus area. Here in the figure, the aorta and vena cava are demonstrated; the para-aortic lymph nodes are removed. Interaortocaval and paracaval lymph nodes are ready for excision (Fig. 9.4).
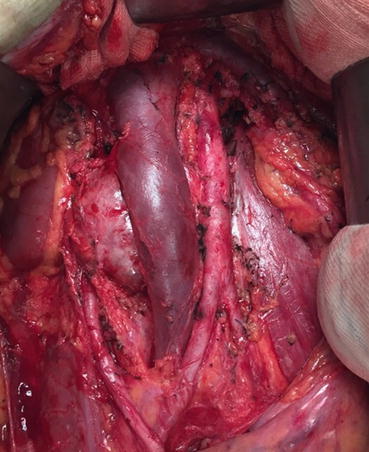

Fig. 9.4
Paracaval and interaortacaval lymph nodes ready for excision
9.7 Minimally Invasive PC-RPLND
Laparoscopic RPLND (L-RPLND) has been restricted since desmoplastic reaction caused by chemotherapy. Besides, instead of mass resection, bilateral templates are recommended techniques for RPLND that makes it much more complicated for laparoscopy. But it could be performed with acceptable results in low-stage tumors in experienced hands [51–53]. In a study including only seven patients, two cases were converted to open surgery, and three had major complications [54]. Rassweiler et al. reported seven conversions to open surgery in nine L-PC-RPLND cases [55]. As a result, L-PC-RPLND is not a self-proven technique and could only be done in large centers with experienced hands [56]. Robot-assisted primary RPLND is reported in limited number of cases [57].
9.8 Complications
Chemotherapy distorts the surgical plane between lymph nodes and surrounding tissues by causing desmoplastic reactions. In order to have a complete resection, there is 20–33% risk of additional procedure implementation that generally includes major vessel repair and nephrectomy [44, 58–61] (Fig. 9.5). Other additional surgeries are splenectomy, pancreatic resection, hepatic resection, and bowel resection with or without stoma. Nah et al. reported that of 848 PC-RPLND cases, 19% had en bloc nephrectomy, and of all 73% had prerenal structural involvement [61]. Nephrectomy risk is correlated with residual mass size and advanced stage disease.
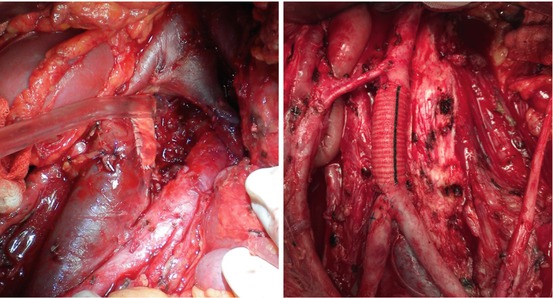

Fig. 9.5
Vena cava patch on left side and aortic graft distal to the inferior mesenteric artery on right side
Some studies reported that PC-RPLND compared with primary RPLND has longer surgical time, more bleeding, longer hospitalization time, and more additional procedure including nephrectomy, caval repair, bowel resection, and thrombectomy [62, 63]. These results are related mainly to the desmoplastic reaction; besides, primary RPLND is done in patients with lower stages.
Baniel et al. defined 144 complications established in 125 cases in their series of 603 PC-RPLND patients (20.7%). Of all, 93% had residual mass ≥ 5 cm, and overall mortality rate was 0.8% [64]. Pulmonary complications were most common because of bleomycin-related pulmonary toxicity and additional pulmonary procedures. Cary et al. reported 22.1% additional procedure and 3.7% complication rate [65]. Additional procedure rate was associated with mass size, high serum markers, and RPLND pathology.
Bleomycin toxicity-related ARDS is a mortal complication [66]. Another complication is chile ascites generally seen along with caval resection [67]. Meticulous clipping or ligation of lymphatic vessels is the most important approach to prevent this complication. Percutaneous drainage is required for symptomatic lymphoceles like compression-related hydronephrosis, bowel obstruction, pain, or infection.
In post-chemo seminomas incomplete resection rate is high due to the difficult dissection caused by the intense desmoplastic reaction [30, 68, 69]. In a study including 97 seminoma and 1269 NSGCT cases, additional procedure and complication rates were 38.1 and 24.7% in seminoma and 26.8 and 20.3% in NSGCTs [68].
The most important care to prevent complications is to perform surgery in experienced centers by expert hands. In a study of 993 patients who underwent PC-RPLND in different centers, those who were operated in higher volume centers had less complications, more transfusion rates, and higher cost [70]. These results may be attributed to the fact that more complex cases are seen in higher volume centers. Mosharafa et al. compared his PC-RPLND results in different time periods and reported that patients who underwent the procedure between 2000 and 2002 had fewer complications, fewer additional procedures, and shorter hospital stay than in the period between 1990 and 1992 [56].
9.9 PC-RPLND Pathology
PC-RPLND pathology has paramount importance for the prognosis and adjuvant treatment setting. The presence of viable tumor or teratoma in RPLND specimen is detected in about 50% of all cases. Carver et al. reported 49% fibrosis, 11% viable tumor (±teratoma), and 39% only teratoma in his 504 NSGCT cases [6]. Current literature reveals the rates of necrosis/fibrosis, viable malignancy (±teratoma), and only teratoma were identified in 25–52%, 9–31%, and 27–67%, respectively, in PC-RPLND pathology [26, 71–74]. Fizazi et al. studied prognostic factors in 238 viable tumor cases [9] and have shown that IGCCCG intermediate- or high-risk group , >10% viable tumor, and incomplete resection were independent poor prognostic factors. 5-year progression-free survival (PFS) rates for patients with no risk factors, with one risk factor, and with two or more risk factors were 100, 83, and 51%, respectively. Although the PFS was better in patients who received postoperative chemotherapy compared to the patients who had not, there was no significant difference in 5-year OS rates. These results were verified by a contemporary study published in 2008 [10]. With a median follow-up of 5.4 years, it was clear that postoperative chemotherapy patients did not have better OS compared to the patients under surveillance and treatment at relapse.
Fox et al. reported viable tumor was detected in 43 of 417 PC-RPLND cases [75]. Of 34 patients who had complete resection, 27 had adjuvant chemotherapy and 7 did not. The patients who had adjuvant chemo had 70% survival; however, all the patients who didn’t have chemo recurred in a median 84-month follow-up.
After primary chemotherapy, 34 of 43 had complete resections, and 27 of the 34 received postoperative cisplatin-based chemotherapy. Nineteen of 27 (70%) were continuously disease-free. All seven who received no postoperative chemotherapy have relapsed.
There is no need for additional treatment in case of teratoma in PC-RPLND. But it usually recurs as mature/immature teratoma or sarcomatoid tumor , which usually has poor prognosis [71, 76]. Growing teratoma syndrome should be considered in case of negative tumor markers with enlarging residual mass during chemotherapy. Patients with incomplete resection in PC-RPLND have higher recurrence rates [77].
9.10 RPLND After Salvage Chemotherapy
Although the data about the role of RPLND in patients receiving salvage chemotherapy is conflicting, evidence suggests that complete resection would always cause finest staging and improved survival. Of all, 10% of advanced GCTs fail to respond primary chemotherapy [72, 74]. Viable tumor rates of patients who underwent RPLND after salvage chemotherapy were reported to be 50% [71, 75, 78]. Complete resection is possible in about 50–70% of cases, and 5-year survival rate is roughly 45–60% [28, 75, 79, 80].
Eggener et al. published the results of 71 patients who underwent RPLND after two or more chemotherapy regimens [80]. Viable tumor, teratoma, and fibrosis rates were 28, 21, and 51%, respectively. Patients who received taxane-containing salvage chemotherapy regimens had lower rates of viable tumor (14 vs. 42%; p = 0.01), higher rates of fibrosis (63 vs. 39%; p = 0.04), and similar rates of teratoma compared to regimens without taxane. It was found that second-line taxane-containing chemotherapy reduces viable tumor rates. The presence of large retroperitoneal mass (≥5 cm) and viable tumor were predictors of worse 10-year disease-specific survival (DSS) (70%). All these results support RPLND—with complete resection done as possible—in select patients after salvage chemotherapy.
9.11 Desperation Surgery
Desperation surgery is performed when tumor markers are rising in spite of chemotherapy. It’s considered an important treatment choice especially in patients with retroperitoneal resectable disease without other visceral metastasis. Albers et al. reported overall persistent viable cancer, and teratomatous elements were identified in 64 and 11% of cases, respectively, in 30 patients [81]. Beck et al. performed desperation surgery in 114 patients with persistent serum tumor markers after second-line chemotherapy. Elevated markers, redo RPLND, and germ cell cancer in the resected specimen were identified as poor prognostic factors. 5-year overall survival was reported as 54% [82]. As a conclusion, surgery has critical importance especially in patients with solitary resectable tumors and rising markers despite salvage chemotherapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree