Clinical potential
References
Detection and differential diagnosis
+
Biopsy guidance
+++
Tumor extent
+++
Grading of gliomas (static amino acid PET)
+
Grading of gliomas (dynamic FET PET)
++
Prognosis
+++
Malignant transformation
+++
Resection planning
+++
Radiotherapy planning
++
Detection of recurrence
+++
Therapy monitoring
+++
15.2 Radiopharmaceuticals for PET in Low-Grade Gliomas
Today, the most widely used application of PET is the measurement of glucose metabolism with FDG in various types of cancer. In cerebral gliomas, FDG uptake is correlated with the degree of malignancy of the tumor (WHO grading) and with the patient’s outcome [4, 13, 14]. Due to the high rate of glucose metabolism especially in the grey matter of the brain, however, it is difficult to distinguish glioma tissue from normal brain tissue by FDG-PET. While most high-grade gliomas WHO grade III and nearly all grade IV glioblastomas show an increased FDG uptake compared to the white matter, low-grade gliomas WHO grade II usually exhibit an indifferent or even a decreased FDG uptake (see Fig. 15.1). Therefore, FDG PET is not useful to delineate low-grade gliomas from the surrounding brain tissue.
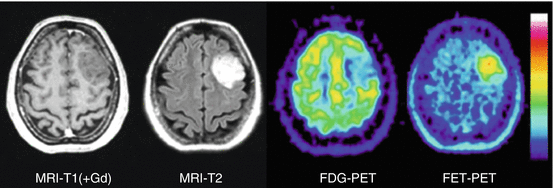

Fig. 15.1
Astrocytoma WHO Grade II in the left hemisphere. The T1-weighted MRI after application of Gd-DTPA shows no contrast enhancement indicating an intact BBB and depiction of the tumor in the T2 weighted MRI is similar. FDG PET shows hypometabolism and is not helpful to guide biopsy. FET PET identifies a hot spot within the tumor and detects an optimal biopsy site
Nevertheless, FDG PET has been shown to be useful to detect malignant transformation in low-grade gliomas and may therefore be useful for follow-up in low-grade gliomas [4]. The use of proliferation markers such as [18F]3′-deoxy-3′-fluorothymidine (FLT) showed even a better correlation with the grade of malignancy and prognosis of cerebral gliomas than FDG uptake or MR spectroscopy [15, 16]. An image-guided biopsy study demonstrated that FLT is a useful marker of cellular proliferation that correlates with regional variation in cellular proliferation, but was unable to identify the margin of gliomas [17]. This is caused by the fact that FLT is not able to pass the intact BBB and accumulates usually in areas with contrast enhancement on MRI only [15, 18–20]. Therefore, portions of the tumor with an intact blood-brain barrier (BBB)—frequently present in low-grade gliomas—cannot be detected with FLT-PET. Furthermore, 11C-Choline or 18F-Fluoro-choline (FCH) has been used as a marker of cell membrane phospholipids in brain tumors and shows a significant correlation of uptake with the degree of malignancy in gliomas [21, 22]. Tracer uptake in areas with intact BBB is generally low but some studies have reported that FCH might be helpful to detect recurrent LGG in brain areas showing no contrast enhancement in MRI [23, 24].
Another interesting approach is to investigate the presence of intratumoral hypoxia using 18F-Fluoromisonidazole [25–28]. Hypoxia in tumors is a pathophysiological consequence of structurally and functionally disturbed angiogenesis along with deterioration in the inability of oxygen to diffuse through tissues. A PET study in patients with cerebral gliomas demonstrated areas of hypoxia in glioblastomas, but all investigated low-grade gliomas showed low uptake of 18F-Fluoromisonidazole [29]. This is not unexpected since tumor growth and angiogenesis in low-grade gliomas are still in a balance so that this approach is particularly attractive for the evaluation of high-grade gliomas.
A promising new target for brain tumor imaging is the mitochondrial translocator protein (TSPO), which is a component of the mitochondrial permeability transition pore and is strongly expressed by glioma cell lines [30]. In the recent past, PET imaging using TSPO ligands such as 11C-(R)PK11195 focused mainly on inflammatory brain diseases as an indicator of microglial activation but recent studies suggest a role of this method in the assessment of brain tumors [31–33]. A recent study has shown that TSPO expression may extend beyond the tumor margins in MRI and amino acid PET indicating an infiltration zone that exhibited tumor progression in the further follow-up of the patients [34].
At present, the best established PET tracers for the investigation of low-grade gliomas are radiolabeled amino acids of the class of large neutral amino acids such as [Methyl-11C]-L-methionine (MET), O-(2-[18F]fluorethyl)-L-tyrosine (FET) and 3,4-dihydroxy-6-[18F]fluoro-phenylalanine (FDOPA) [5, 6, 9–12, 35, 36]. Because the uptake of these amino acids by both, the white and grey matter of normal brain tissue is relatively low, cerebral gliomas can be distinguished from the surrounding normal tissue with high contrast. It was long assumed that increased uptake of MET in brain tumors reflects an increased protein synthesis rate. Experiments in mice, however, demonstrated that an inhibition of protein synthesis did not influence the uptake of radiolabeled MET in tumors and brain [37] suggesting that alterations of amino acid transport rather than increased protein synthesis caused increased uptake in tumors. Furthermore, the predominant role of transport phenomena for increased amino acid uptake in gliomas is confirmed by the observation that PET using radiolabeled amino acids such as FET which are not incorporated into protein exhibit nearly identical results concerning brain tumor imaging as MET PET or FDOPA PET. Thus, a number of studies have shown that imaging of cerebral gliomas with MET, FET and FDOPA is rather similar [38–43]. Since FDOPA is a precursor of dopamine it shows also uptake in the striatum and can be used to trace the dopaminergic pathway in the nigrostriatal region to evaluate the presynaptic function in patients with neurodegenerative and movement disorders [44]. This property may cause problems in the delineation of gliomas affecting the striatum [12, 45].
The increased uptake of amino acids such as MET, FET and FDOPA by cerebral glioma tissue appears to be caused predominantly by increased transport via the transport system L for large neutral amino acids namely the subtypes LAT1 and LAT2 [46–50]. A recent study suggested that the trapping of FET within the cells is caused by the asymmetry of its intra- and extracellular recognition by LAT1 [48]. Nevertheless, there appear to be some differences in transport characteristics of MET, FET and FDOPA. FET shows different patterns of time-activity-curves in low-grade and high-grade gliomas [51–55] which could not be observed with MET or FDOPA [43, 56].
Since large neutral amino acids also enter normal brain tissue, a disruption of the BBB, i.e., enhancement of contrast media on MRI scans, is not a prerequisite for intratumoral accumulation of MET, FET and FDOPA (see Fig. 15.1). Consequently, uptake of these tracers has been reported in many low-grade gliomas without BBB leakage [5, 35, 57–60].
Most PET studies of cerebral gliomas have been performed with the amino acid MET [5], although the short half-life of 11C (20 min) limits the use of this technique to the few centers that are equipped with an in-house cyclotron facility. In contrast to MET, 18F-labelled amino acids (half-life, 109 min) such as FET and FDOPA can be transported from a cyclotron unit to multiple external PET centers. This enables a wider application of amino acid PET in clinical diagnosis. One of the best-established 18F-labelled amino acids is FET that can be produced in large amounts for clinical purposes like the widely used FDG [36, 61, 62].
Animal experiments have shown that FET, in contrast to MET, exhibits no uptake in inflammatory cells and in inflammatory lymph nodes but false positive uptake has been observed for MET, FET and FDOPA in human brain abscesses, demyelinating processes, near cerebral ischemia and hematomas [12, 63–65]. Therefore, increased uptake of the tracers is not specific for cerebral gliomas although increased amino acid uptake has a high positive predictive value for cerebral gliomas [66]. The report is focused on the clinical experiences with MET, FET and FDOPA, which are at present the best-validated amino acid tracers for PET.
15.3 Clinical Applications of PET in Diffuse Low-Grade Gliomas
15.3.1 Detection of Low-Grade Gliomas and Differential Diagnosis
The diagnostic potential of amino acid PET to detect low-grade gliomas is limited since MET and FET exhibit increased uptake only in a fraction of about 60–80% of low-grade gliomas [6, 35, 57, 58, 60, 63, 65, 67–69]. The specificity of MET, FET and FDOPA PET for neoplastic lesions and especially in the case of low-grade gliomas is generally affected by possible tracer uptake in the area of benign processes such as hematoma, ischemia, traumatic brain injury, and acute inflammatory processes [5, 63–65, 70–73]. In the largest study to date evaluating MET PET in a consecutive series of 196 patients with suspected brain tumors, differentiation between gliomas and non-neoplastic lesions was correct in 79% using a threshold of the tumor/brain ratio of 1.47. Exclusion of high-grade gliomas (99 low-grade gliomas versus 24 non-neoplastic lesions) yielded a sensitivity of 67% and specificity of 72% for distinguishing low-grade gliomas from non-neoplastic brain lesions [6, 68]. The diagnostic performance of FET PET has been evaluated in a series of 174 newly diagnosed cerebral lesions with suspicion of glioma, which included 72 histologically confirmed diffuse low-grade gliomas [58]. In that study, the mean tumor to brain ratio (TBR) was 1.8 ± 0.5 in low-grade gliomas versus 1.4 ± 0.4 in nonneoplastic lesions yielding a sensitivity of 79% and specificity of 48% for distinguishing low-grade gliomas from non-neoplastic brain lesions. These results are similar to the observations in other publications in which low-grade gliomas, with the exception of oligodendrogliomas, presented with mean TBR in the range of inflammatory and other active (e.g., ischemic, traumatic) brain lesions [63, 65, 67]. Higher amino acid uptake in the subgroup of low-grade oligodendrogliomas and oligoastrocytomas according to the WHO classification 2007 [74] has been reported in several studies and is most probably related to the increased cellular and vascular density in this glioma subtype [52, 60, 75–77].
Taken together, the diagnostic accuracy of amino acid PET to detect low-grade gliomas and to differentiate suspicious lesions from non-specific uptake in non-neoplastic lesions is limited. Therefore, a histological evaluation of suspicious brain lesions by biopsy remains necessary under most circumstances.
15.3.2 Identification of an Optimal Site for Biopsy
An important aspect of the diagnostic assessment of low-grade gliomas is the definition of areas with the highest cellular proliferation rates. Since the tumor biology is dominated by the most aggressive glioma parts, representative tissue samples are vitally important for histological tumor diagnosis, prognostication, and treatment planning. The ability of MRI to show the most rapidly proliferating portions of diffuse low-grade gliomas is limited, particularly when the tumor shows no contrast enhancement in MRI, which occurs frequently in low-grade gliomas. FDG and FLT PET are usually negative in low-grade gliomas and provide no information on regional heterogeneity of metabolic activity in these tumors. Radiolabeled amino acids exhibit increased uptake in the majority of diffuse low-grade gliomas and are helpful to optimize the targeting of biopsies and prevent the problem of non-diagnostic biopsies from non-specifically altered tissue (Figs. 15.1 and 15.2). Biopsy controlled studies have shown that MET and FET uptake correlate with microvessel and cell density in non-contrast enhancing gliomas [77–79]. Vascular density is a frequently described feature linked to early malignant transformation in gliomas [80]. A number of studies have compared the diagnostic potential of PET using FDG and MET or FET to identify metabolic hot spots in cerebral gliomas to guide biopsy [67, 81, 82]. These studies consistently report that regionally increased FDG uptake, if present, is congruent with that of increased MET or FET uptake but amino acid PET is generally more sensitive than FDG PET. A number of studies have evaluated the role of amino acid PET for biopsy guidance in the subgroup of low-grade gliomas. In a study with 32 patients that included 10 low-grade gliomas MET PET allowed the definition of a biopsy target in all low-grade gliomas while FDG showed increased uptake in only one of these tumors [83]. In a patient series of 22 histologically confirmed low-grade gliomas, FET PET identified a local maximum for biopsy guidance in 16 of the tumors (72%), while FDG identified a metabolic spot in only 2 (9%) of the low-grade gliomas [67]. Another study including 72 histologically confirmed diffuse low-grade gliomas, FET PET identified a local maximum in 79% of the tumors [58]. Other studies emphasize the role of kinetic analyses of FET uptake in low grade glioma [51, 54, 59, 69, 84]. Areas with an early peak in FET uptake followed by a descending time activity curve were associated with areas of malignant transformation and poor prognosis. Interestingly, a “malignant curve pattern” was also predictive for poor outcome if FET uptake in the suspicious brain lesion was low [51, 59, 69]. These data suggest that amino acid PET is a useful tool for identifying metabolic hot spots in low-grade gliomas to target biopsies. Furthermore, dynamic FET PET appears to provide important additional information on the aggressiveness of the tumors independent of the degree of tracer uptake. Nevertheless, it is not yet proven beyond doubt that the maximum concentration of amino acid uptake in low-grade gliomas corresponds to the most aggressive part of the tumor and further studies are needed to investigate this aspect (Fig. 15.3).
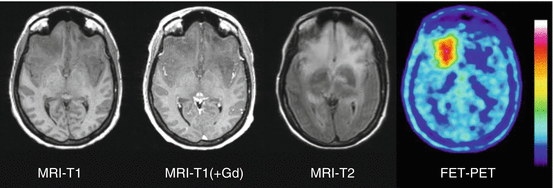
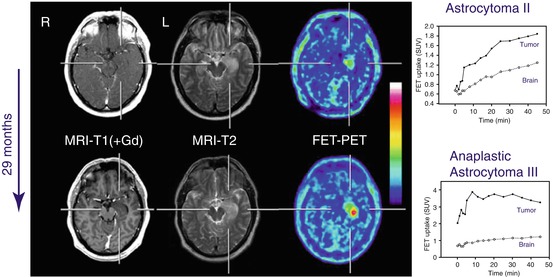

Fig. 15.2
Astrocytoma WHO Grade II in the frontal lobe. T1-weighted MRI after application of Gd-DTPA shows no pathological contrast-enhancement and a tumor cannot be clearly delineated. T2-weighted MRI shows widespread abnormalities within the complete frontal lobe and is not helpful to depict the tumor. FET PET identifies a clear tumor with high tracer uptake in the lower frontal lobe

Fig. 15.3
MRI and FET PET and of a patient with newly diagnosed histologically confirmed diffuse astrocytoma WHO grade II in right temporal lobe (upper row). T1 weighted MRI shows no contrast enhancement (left) and hyperintensity on T2-weighted image (middle) and slightly increased FET uptake (left). Time–activity curve on the right shows constantly increasing FET uptake. Twenty-nine months later (lower row), patient presented with malignant progression to WHO grade III astrocytoma associated with significant increase in FET uptake, discrete contrast enhancement and enlargement of hyperintensity on T2-weighted image. Dynamic evaluation of FET uptake (right) shows a “malignant curve pattern” with an early peak of FET uptake followed by declining time activity curve
15.3.3 Delineation of Tumor Extent for Treatment Planning
Multiple studies in which the radiological findings were compared with the histological findings in tissue samples obtained by biopsy or open surgery have provided clear evidence that PET using radiolabeled amino acid detects the solid tumor mass of cerebral glioma tissue more reliably than either CT or MRI [81, 83, 85–88]. This is especially relevant for the non-enhancing parts of gliomas in MRI, which predominantly occur in low-grade gliomas. In a study exploring the potential of FET PET to image the extent of cerebral gliomas, 52 neuronavigated biopsies were taken from cerebral gliomas of 31 patients. Neoplastic tissue was found in 94% of biopsies in FET-PET positive areas, but only in 53% of the suspicious areas identified by MRI [87]. In that study, 12 biopsies yielded the histopathological diagnosis of a diffuse low-grade glioma and FET uptake was increased in all but one of the areas from which the biopsies were taken. In contrast, none of these areas showed contrast-enhancement on MRI. Another study investigated the role of FET-PET as a surrogate marker for accumulation of 5-aminolevulinic acid (5-ALA), which is used as a metabolic marker of malignant glioma cells for fluorescence-guided resection [89]. In that study, patients with 17 low-grade gliomas were included. FET was positive in 7 of the tumors while 5-ALA was observed in only one of the low-grade gliomas, which showed corresponding contrast enhancement. These data indicate that amino acid uptake in PET is a more sensitive indicator of low-grade glioma than 5-ALA fluorescence.
Since amino acid PET appears to be a valuable instrument to detect the solid tumor mass of cerebral gliomas, this technique has been used for resection planning. In a study evaluating integrated MET PET and MRI guided resection of 103 brain tumors, a large fraction of low-grade gliomas was included [90]. Resection planning in 59 low-grade gliomas demonstrated that the PET volume did not match the MR volume and improved the tumor volume definition in 88% of the cases. Similar results were reported in other studies for MET and FET PET which mainly included high-grade gliomas [91–93].
These data suggest that resection of low-grade gliomas guided by amino acid PET may increase the resection extent and thus the patients’ survival. It needs, however, to be considered that MET, FET and FDOPA show increased uptake in only 60–80% of low-grade gliomas and that the resection of the tumors with low amino acid uptake cannot be improved.
The improved imaging of glioma tissue using amino acid PET has also been applied to improve planning of radiation treatment of brain tumors [94]. A number of centers have started to integrate amino acid imaging into CT- and MRI-based radiotherapy planning, particularly in high-grade gliomas and when high-precision radiotherapy is to be given or in the setting of dose escalation studies or for the re-irradiation of recurrent tumors [95–103]. Experiences with amino acid PET radiotherapy planning of low-grade gliomas are limited but indicate improved sensitivity in detecting postoperative residual tumor and a benefit for radiotherapy planning in patients with inconclusive MRI findings [104]. Improved outcome of the patients with radiotherapy planning by amino acid imaging compared with conventional therapy planning, however, has not yet been proven.
15.3.4 Glioma Grading and Prognosis
FDG PET is considered as a relative accurate predictor of tumor grade and prognosis of cerebral gliomas and the detection of foci with increased FDG uptake in low-grade glioma is highly suspicious for malignant progression [13, 105]. The combination of FDG PET and multiparametric MRI may further improve the diagnostic accuracy to differentiate high-grade and low-grade glioma [14]. Most PET studies employing amino acids have shown that gliomas of different WHO grades overlap in their degree of amino acid uptake, so that the tumor grade cannot be reliably predicted with this technique [5, 19, 41, 52, 58, 87, 106]. A high potential to differentiate high-grade and low-grade gliomas has also been claimed for FLT, but FLT uptake goes along with BBB disruption and there is a high fraction of anaplastic astrocytoma without significant contrast enhancement on MRI which consecutively are negative in FLT PET [19, 20].
Using FET PET a number of studies have demonstrated that the evaluation of tracer kinetics in the tumors may be helpful to differentiate between high-grade and low-grade gliomas [52, 53, 55, 59, 107]. High-grade gliomas are characterized by an early peak of the time-activity curve around 10–15 min after tracer injection followed by a decrease of FET uptake. In contrast, time–activity curves slightly and steadily increase in low-grade gliomas of WHO grade II. Using dynamic evaluation of selected regions of the tumor, high-grade and low-grade gliomas could be distinguished with an accuracy of 70–90% in primary tumors as well as in recurrent tumors [52, 53, 59, 84, 107]. Furthermore, a recent study suggested that early static scans of FET uptake have a higher diagnostic accuracy for grading of gliomas than the standard 20–40 min scans but this approach did not reach the accuracy of dynamic FET imaging [108]. Studies using MET and FDOPA demonstrated that unlike FET PET, the time-activity curves of tracer uptake do not allow the classification of low- and high-grade gliomas [43, 56].
Considering gliomas of all WHO grades the prognostic significance of amino acid uptake remains a matter of controversy. Some studies seem to show that lower amino acid uptake especially in astrocytic glioma is associated with a better prognosis, but there is generally high uptake in oligodendroglioma despite their apparently better prognosis [5, 60, 75, 76, 109]. Recent studies suggest that dynamic FET PET [110], the “biological tumor volume” (BTV) as assessed by amino acid PET at primary diagnosis [111, 112] or textural parameters considering tumor heterogeneity may be important predictors of prognosis [113].
For patients with low-grade gliomas prognostication is an important factor for disease management. Some of these patients have a stable course with an excellent quality of life for many years or decades even without treatment, while others experience rapid tumor progression with malignant transformation to a high-grade glioma and a poor prognosis. A better identification of individuals with either a poor or a favorable prognosis is highly desirable to optimize patient counseling. A study with MET PET showed that these patients benefit from a surgical procedure only when increased amino acid uptake can be demonstrated [114]. In a series of 24 patients with low-grade gliomas, patients with a tumor/brain ratio >2.2 had a significantly shorter survival time than the patients with a tumor/brain ratio <2.2 [115]. Similarly, in a series of 50 patients with low-grade gliomas a SUV of FDOPA uptake >1.75 was an independent predictor of disease progression [35]. Another study indicated that the combined evaluation of FET-PET and MR morphology was a statistically significant prognostic predictor for patients with newly diagnosed low-grade gliomas [57]. Within a 7-year period, a group of 33 consecutive patients with previously untreated non-enhancing WHO grade II glioma were included in a prospective study. A baseline, both MRI and FET-PET were performed before histology in all patients on tissue samples by biopsy and a “watch and wait” strategy without further treatment was started. During the follow-up it turned out, that baseline FET uptake and a circumscribed versus a diffuse growth pattern on MRI were highly significant predictors for patients course and outcome: Those low-grade gliomas that were well delineated on MRI and showed no FET uptake had an excellent prognosis with long progression-free intervals, good clinical condition and late malignant transformation. In contrast, patients with low-grade gliomas with diffuse tumor margins on T2-weighted MRI and FET uptake had a poor outcome with early progression in combination with malignant transformation to a HGG, rapid clinical deterioration, and die earlier. A recent study in 54 gliomas of WHO grade II observed no correlation between FET uptake and progression-free survival but that analysis included 16 patients with recurrent tumors and comparison with other studies is difficult [60]. In any case, also low-grade gliomas with low FET uptake should be monitored carefully because also tumors without tracer uptake can harbor high-grade glioma tissue [59].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree