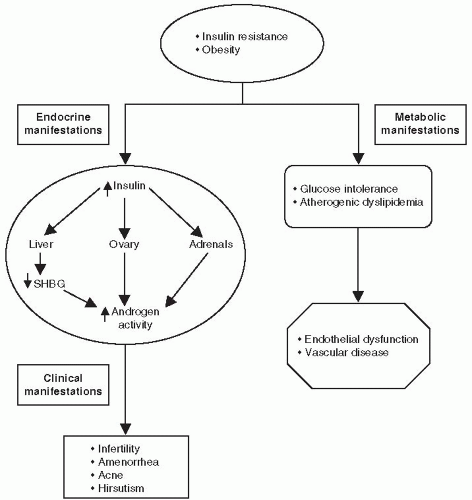
LH hypersecretion is a characteristic hallmark of PCOS. LH is secreted in a pulsatile manner. Women with PCOS have an increase in both the LH pulse frequency and amplitude resulting in increased 24-hour secretion. LH hypersecretion is thought to occur secondary to an increased frequency of hypothalamic gonadotropin-releasing hormone (GnRH) pulsation. Higher circulation levels of LH stimulate the production of androgens by the ovarian theca cells. (GnRH) must be accelerated in PCOS.9
Increasing the GnRH pulse generator favors the synthesis and release of LH over FSH.
When the concentration of LH increases relative to FSH, the ovaries preferentially synthesize testosterone.
Insulin acts synergistically with LH to increase androgen production within the theca cell.
Insulin also inhibits the hepatic synthesis of SHBG, which normally binds testosterone. The higher levels of unbound or “free” testosterone increase the biologic activity of the circulating hormone. The concentration of free testosterone is often elevated, whereas the total testosterone level may be only slightly increased.10
Testosterone further inhibits (whereas estrogen stimulates) the hepatic synthesis of SHBG.11
with the hypothalamic-pituitary axis, leading to anovulation (Fig. 4-1). The absence of a dominant follicle prevents development of nondominant follicles, resulting in the formation of multiple ovarian cysts.12 Androgen excess also affects other metabolic parameters such as lipid concentrations. A recently published study demonstrated reduced levels of glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) in patients with PCOS.13 In normal subjects, these incretin hormones are responsible for 70% of the insulin response during a meal. However, in patients with T2DM and in other insulin-resistance conditions, the incretin effect is impaired. The reduced physiologic levels of incretin hormones in patients with PCOS may have pharmacologic and therapeutic implications warranting future investigations.
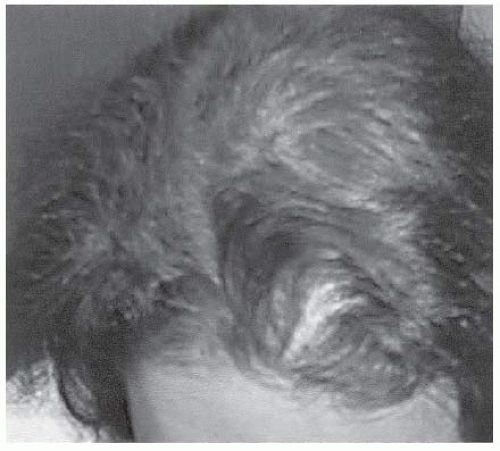
A history of irregular menstrual cycles and anovulation with onset at puberty. Twenty-five percent of women with PCOS have regular menstrual cycles.
Elevated total and free testosterone levels.
The presence of polycystic ovaries and the exclusion of other hormonal disorders with similar clinical features such as adult-onset congenital adrenal hyperplasia, hyperprolactinemia, adrenal or ovarian androgen-producing adenomas, hyperthecosis, and Cushing syndrome. The term hyperthecosis refers to the presence of nests of luteinized theca cells in the ovarian stroma secondary to differentiation of the ovarian interstitial cells into steroidogenically active luteinized stromal cells. These nests or islands of luteinized theca cells are scattered throughout the stroma of the ovary, rather than being confined to areas around cystic follicles as in PCOS. The end result is greater androgen production of androgens. The clinical features of hyperthecosis are similar to those of PCOS. However, women with hyperthecosis have more hirsutism and are much more likely to be virilized.16 Hyperthecosis results in elevations of both estrogen and androgen production, increasing the risk of endometrial hyperplasia and endometrial carcinoma, especially in postmenopausal women.
TABLE 4-1. Clinical Manifestations of PCOS | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
iron deposits in pancreatic islets similar to mechanistic models observed in hemochromatosis. The use of insulin sensitizers, such as metformin, might be useful in reversing elevated iron stores.22a
risk of developing T2DM at either end of the distribution of sleep duration and with qualitative disturbances of sleep.” Subjects averaging less than 5 to 6 hours per night of sleep have a 28% chance of developing T2DM, whereas those with difficulty maintaining sleep have an 84% likelihood of developing the disorder.
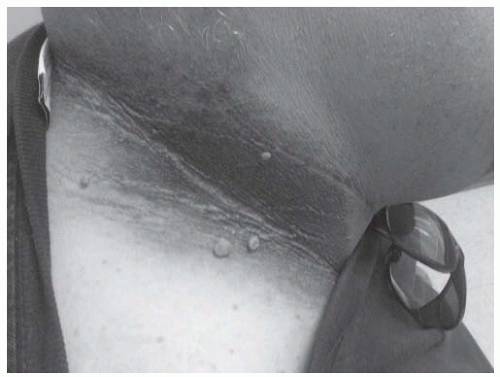
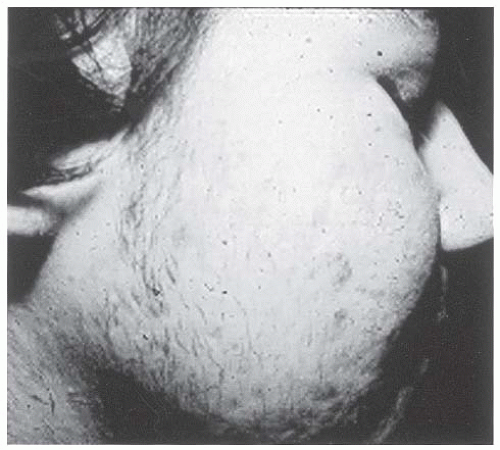 Figure 4-5 • Severe facial acne and hirsutism in a patient with PCOS. (Photo provided courtesy of Walter Futterweit, MD.) |
that provides information related to one’s energy expenditure to hypothalamic regulatory centers. In humans, circulating leptin levels rapidly decrease or increase in response to acute caloric shortage or surplus. Elevated plasma leptin levels decreases hunger and vice versa. Ghrelin is produced predominantly by the stomach and is also involved in energy balance regulation. However, in contrast to leptin, elevation in plasma ghrelin stimulates appetite. (See Chapter 8, Comanaging Associated Disorders of Diabetes).26,26a Individuals with insomnia, sleep fragmentation, and sleep apnea have a physiologic state that favors ghrelin secretion and leptin resistance. As the body spends more time in the “awake state,” additional nutritional intake and fat storage are needed to insure an adequate energy source for 20 hours of activity. Individuals who sleep 7 to 8 hours per night require enough energy intake and storage for only 16 to 17 hours of daily activity. The short night sleep patterns associated with insomnia, sleep fragmentation, and obstructive sleep apnea increase ghrelin secretion and favors leptin resistance. Although an individual may remain in bed for 10 to 12 hours a day, he or she is not sleeping efficiently. Ghrelin levels rise as the body strives to provide additional nutritional and energy stores to be used for during the 20 hours per day of wakefulness. The tendency for these patients is toward weight gain and insulin resistance. CPAP (continuous positive airway pressure) usage in young, obese patients with PCOS and sleep apnea has been found to improve insulin sensitivity, reduce diastolic blood pressure, and reduce sympathetic output. Patients with a BMI > 35 kg per m2 who used CPAP for 8 hours per night demonstrated the best improvement in metabolic parameters.26b
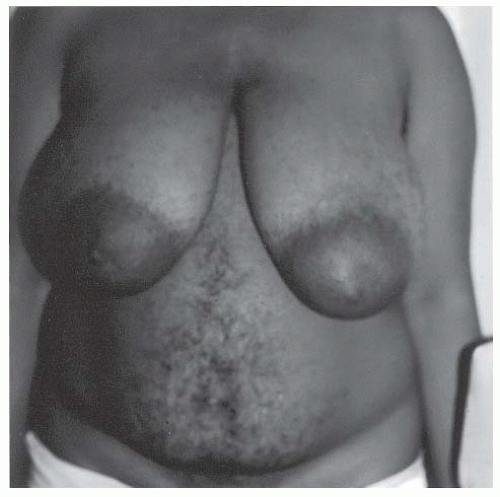
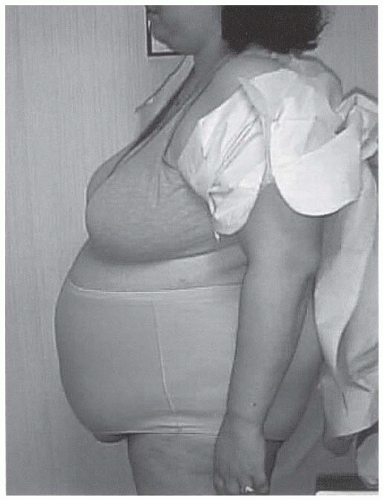 Figure 4-7 • Central obesity, a hallmark of PCOS and insulin resistance, is strongly associated with CVD risk. (Photo provided courtesy of Walter Futterweit, MD.) |
for adolescents and older women have not been determined. Notwithstanding these limitations, measurement of the free testosterone is thought to be a sensitive method of assessing hyperandrogenemia.30 A total testosterone value greater than 50 ng per dL is considered elevated.31 The optimal value for serum testosterone in women is unknown. Most women with a level greater than 50 ng per dL will have irregular menses and clinical symptoms related to hyperandrogenism. The level of free (unbound) testosterone is usually elevated in patients with PCOS.
TABLE 4-2. Interpretation of Laboratory and Radiographic Studies in Patients Suspected of Having PCOS | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree