Pleural malignant disease is a heterogeneous group of cancers that range from primary intrathoracic cancers such as mesothelioma to metastatic cancers from extrathoracic sources. Often found incidentally, pleural malignancies may present as small pleural effusions or asymmetric pleural thickening on a chest x-ray or CT scan. As disease progresses, tumor may decrease lung compliance by entrapment and fluid production causing pressure on other structures, leading to symptoms of chest pain, dyspnea, and cough. Goals of care depend on the type of malignancy (primary vs. metastatic) and the ability to control disease (small volume vs. large volume). Curative treatment may be possible in early-stage primary pleural cancers or in select cases of metastatic metastases, but often palliation is the true goal for most patients with pleural disease.
Malignant pleural effusions (MPEs) are often the first sign of a primary pleural malignancy or advanced extrathoracic cancer. In this section, we focus on MPEs that represent stage IV cancers that have spread to the pleural space and indicate M1 disease. Later in this chapter we will discuss MPEs related to primary mesothelioma. MPEs are extremely common with an estimated 1 million effusions occurring each year in the United States, with up to a quarter of those being malignant.1 The median survival after diagnosis with an MPE is 4 months, with the most common cancers that cause MPEs, lymphoma, lung, breast, ovarian, and gastrointestinal cancers.2 Regardless of the etiology, finding an MPE is a poor prognostic sign and goals of treatment should be focused on palliation.3
The presence of cancer cells in the pleural fluid defines an MPE.2 Cancers may spread to the pleura hematogenously, through lymphatics, or by direct extension. Malignant effusions are associated with an exudative process which is driven by two different mechanisms. First, tumor deposits established along the pleural membrane obstruct lymphatic flow. Second, implants stimulate the release of chemokines that promote increased vascular and pleural permeability allowing the leakage of larger proteins.1
The advancing pathologic process may decrease lung compliance by entrapment and invasion of local structures, leading to the development of chest pain and dyspnea. Other signs include cough, anorexia, and weight loss. Physical exam findings may include decreased breath sounds and dullness to percussion.
The prognosis of patients with MPEs is extremely poor, with a median survival being 4 months.3 This estimate should guide treatment and focus interventions on symptom control.
Every patient with an MPE should undergo a history and physical. The history may identify risk factors such as exposure to occupational hazards, smoking, personal and family history of malignancies, and the presence of constitutional signs and symptoms. The chest exam may identify dullness to percussion, diminished, or absent breath sounds. Other physical exam findings concerning for malignancy include lymphadenopathy, organomegaly, and ascites. While distended neck veins, an S3 gallop or a right ventricular heave may suggest non-neoplastic causes of an effusion such as heart failure.
Imaging is a valuable tool in the diagnosis and treatment of pleural effusions. A standard lateral chest radiograph can identify as little as 50 mL of pleural fluid. Other important radiographic findings include a meniscus sign, mediastinal shift, pleural thickening, or elevation of a hemidiaphragm.2 Ultrasound (US) can also identify small amounts of pleural fluid and pleural thickening, and has the added benefit of evaluating for the invasion of local structures. Importantly, US can be used to guide diagnostic and therapeutic procedures such as thoracentesis, biopsies, and catheter insertion. Cross-sectional imaging is valuable in its ability to identify malignancies of the lung, adrenal glands, liver, and colon. Similar to ultrasonography, computed tomography (CT) is an excellent modality for image-guided procedures. Positron emission tomography (PET) fluorodeoxyglucose (FDG) activity in the pleural space is sensitive in identifying MPEs.4 However, it is important to note that PET findings can be obscured by recent procedures, specifically pleurodesis. Magnetic resonance imaging (MRI) is helpful in evaluating tumor involvement of the chest wall and diaphragm.2
An important step in evaluating any pleural effusion is classifying the fluid as transudative versus exudative. A transudative fluid collection is the result of poorly balanced osmotic pressure across the pleural membrane. This results in increased serum leak across the pleural barrier. The leading causes of transudative effusions include heart failure, cirrhosis, and pulmonary embolism. Malignancy is usually associated with an exudative process driven by obstructed lymphatic flow and increased vascular and pleural permeability.1 Of note, patients with cancer can also develop paraneoplastic effusions that are transudative.
Light’s Criteria represent a widely accepted set of rules used to classify effusions as transudative or exudative (Table 64-1).5,6 If one or more of the criteria are met, then the fluid is considered exudative. The diagnostic accuracy of the criteria is >90.6 When the values are near the thresholds an effusion can be misclassified.2,6 Though not considered a part of the Light’s Criteria, MPEs are also associated with low glucose levels and low pH. It is also important to note that patients with lymphoma can develop chylous effusions with elevated triglyceride and cholesterol levels. The treatment of persistent chylothoraces is drainage and thoracic duct ligation.
Equally important adjuncts include cytology and antigen testing, such as carcinoembryonic antigen (CEA). These tests can differentiate benign effusions from MPEs; however, they often fail to identify the origin of the cancer requiring further testing. Genetic analyses including studies that identify DNA methylation patterns, mutations, and microsatellite instability can be diagnostic. For example, Brock et al7 identified that DNA methylation was present in 29% of patients with MPEs versus 0% in patients with benign effusions. Also, Robinson et al8 documented elevated levels of serum mesothelin-related protein (SMRP) in a significant percentage of patients with mesothelioma, but in relatively few patients with lung cancer. Flow cytometry is also an important modality in lymphoma patients.9
The yield and accuracy of cytologic testing on MPEs is dependent on the sample size and the type of cancer involved. The yield can be as high as 70% for metastatic adenocarcinoma, and as low as 10% for mesothelioma. When fluid analyses are nondiagnostic and the suspicion of malignancy is high an open or closed pleural biopsy is indicated. Blind closed procedures are often low yield; hence, image-guided techniques using US or CT, or operative techniques are preferred. Due to the high diagnostic yield of video-assisted thoracic surgery (VATS) among those with suspected cancer it is the procedure of choice, if the patient can tolerate surgery. VATS provides sufficient biopsy material, allows evaluation of the pleura, and can be combined with therapeutic procedures.2 An open limited thoracotomy is another surgical approach. It is important to note that patients with MPE often present with limited lung function and may not tolerate anesthesia, single lung ventilation, or the induced pneumothorax required for surgical biopsies.
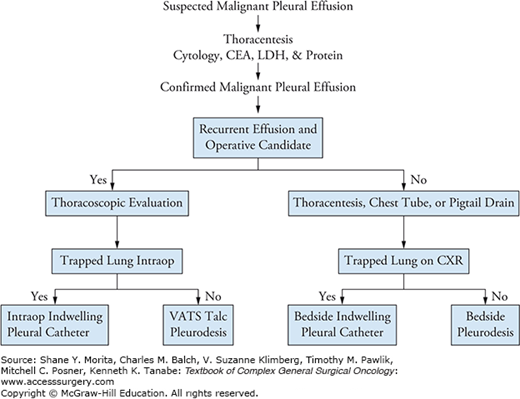
Treatment of MPEs does not improve survival; however, it may effectively palliate symptoms.10,11 Hence, evaluating the fitness and life expectancy of the patient is critical. Fragile patients with small effusions may benefit from conservative management. Interventions include thoracentesis, chest tubes, small bore indwelling catheters, decortication, and pleurodesis. The treatment sequence should be adjusted based on the patient’s short-term prognosis to minimize interventions and to improve quality of life. A proposed algorithm is shown in Figure 64-1.
Thoracentesis with or without image guidance can be therapeutic while providing fluid for analysis. However, there is evidence that 98% to 100% of patients will reaccumulate fluid within 30 days.2 Also, re-expansion pleural edema may not be tolerated in ill cancer patients. Repeated procedures should be reserved for patients with cancers that are likely to respond to treatment or patients that cannot tolerate more invasive procedures.
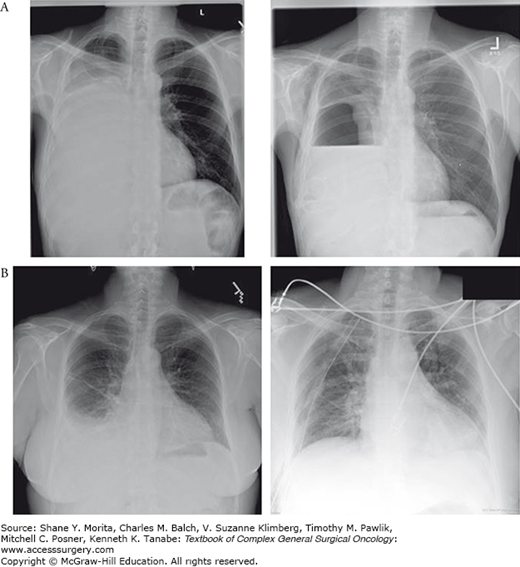
Chest tube placement facilitates drainage and symptom relief. Importantly, a post-placement radiograph can identify if the lung can re-expand or if the lung is “trapped” (Fig. 64-2A). In the setting of a trapped lung the efficacy of decortication is low, given the limited life expectancy and the need often for a thoracotomy to effectively decorticate the lung. Among patients with a trapped lung an indwelling pleural drainage catheter may be the best option to intermittently drain accumulating fluid.12
FIGURE 64-2
Trapped versus nontrapped lung. A. Patient with an MPE and a trapped lung seen before and after drainage of effusion. B. A patient with an MPE with complete re-expansion after drainage. Patient A is a candidate for intermittent thoracentesis or indwelling pleural catheter. Patient B is a candidate for pleurodesis by chest tube or thoracoscopy.
For patients whose lungs expand to occupy the chest cavity (Fig. 64-2B), pleurodesis is an excellent long-term solution. The efficacy of the procedure is reliant on the apposition of the visceral and parietal pleura. Patients with endobronchial lesions, extensive intrapleural tumors, and loculations with trapped lungs are unsuitable candidates.2 Among patients that are likely to respond, pleurodesis can be performed through an indwelling catheter (9 to 14 Fr), chest tube (20 to 32 Fr), or with VATS. Lidocaine can be used to decrease the pleuritic chest pain associated with the procedure. Chemical pleurodesis can be performed utilizing talc, doxycycline, povidone-iodine, or bleomycin. These medications incite an inflammatory reaction between the parietal and visceral pleura resulting in the lung being “stuck” in an expanded state. Mechanical pleurodesis can also be performed by mechanical debridement of the pleura during VATS or open surgery. Systematic reviews have identified that talc has the highest efficacy (up to 93% effective) and is associated with the lowest recurrence rates.13,14 Performing a thoracoscopic talc pleurodesis has been shown to improve effectiveness compared to slurry installation via chest tube.13 Adverse side effects include fever, dyspnea, pain, atelectasis, pneumonia, arrhythmias, empyema, and acute respiratory distress syndrome.10,12 Larger particle size talc (>20 μm) is recommended to avoid systemic side effects.2
Malignant pleural effusions are associated with such a poor prognosis, but as chemotherapy evolved, specifically for sarcomas and breast cancers, metastatic disease to the pleura may be treatable in select cases. If metastatic pleural disease is localized, it may be amenable to surgical resection with the goal of cure. This is separate than an effusion which contacts the entire pleural surface. Decisions on treatment in this setting should only be made in a multidisciplinary setting with input from medical and radiation oncology. Key factors in deciding to operate should include limited pleural or other metastatic disease, patient fitness, and lack of disease progression determined by serial radiologic exams and time.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree