Chapter 16 Khaled El-Ghariani Department of Stem Cells and Therapeutic Apheresis Services, NHS Blood and Transplant and Sheffield Teaching Hospitals NHS Trust, Sheffield, UK, and University of Sheffield, Sheffield, UK Plasma exchange is a therapeutic procedure in which part of the patient’s plasma is replaced with an appropriate alternative fluid. The rationale of such a therapy is that plasma removal would also reduce the body content of a substance or substances that are implicated in the pathogenesis of the disorder being treated. Although plasma exchange is mainly used to reduce the immunoglobulin contents of the human body, to ameliorate an immunologically based disease process, the procedure could also deplete other large-molecular-weight substances such as immune complexes; complement components; coagulation factors, particularly fibrinogen and ultralarge von Willebrand factor (vWF) multimers; and low-density lipoprotein. The efficiency of apheresis removal of a substance depends on its density, its rate of synthesis and its predominant presence in the intravascular rather than extravascular compartment, as well as the speed of equilibrium between these two compartments following plasma exchange [1]. IgM and fibrinogen are relatively easily depleted following repeated plasma exchange, followed by IgG and IgA [2]. Given their rate of synthesis and/or extravascular distribution, other smaller molecules are less effectively removed. The term plasmapheresis (Greek word aphaeresis, meaning to take away) is sometimes used interchangeably with plasma exchange; however, the term plasmapheresis is best used to describe plasma removal (e.g. plasma donation) without replacement fluid, rather than to describe genuine plasma exchange [3]. Plasma exchange can be performed either by centrifugal separation or by membrane filtration. Following carefully adjusted centrifugation, blood separates into layers depending on component density. Red cells, being the most dense, settle on the bottom followed by granulocytes, lymphocytes, platelets and finally plasma on the top [1]. Centrifugal devices often use light sensors to detect the borders of separation between these layers, divert the plasma into discard and infuse the required volume of replacement fluid through the machine’s sophisticated tubing system. This technology usually uses citrate as an extracorporeal anticoagulant, and it is popular in haematology practice because it is versatile and can be used to exchange red cells and to collect peripheral blood stem cells. A commonly used centrifugal device is the COBE® Spectra Apheresis System and the next generation called the Spectra Optia® Apheresis System (TERUMO BCT). The advantage with centrifugation technique is that there is no size limit of the molecules being removed. On the other hand, filtration-based exchange uses membrane separation, usually hollow fibre filtration, and separation is based mainly on particle size. This allows separation of plasma from cellular components. Plasma is removed and replacement fluid is infused into the patient. This system, unlike centrifugation, cannot perform cell collection or exchange [1], and large molecules such as IgM may not pass through the filter and so cannot be efficiently eliminated. This system usually uses heparin as an extracorporeal anticoagulant, and because of its similarities with haemodialysis, it is more commonly used by the renal teams. An example of a filtration device is the HF440 (Infomed blood purification devices). The benefit of filtration method is that a large filter can be easily added to a haemodialysis circuit although the size of the molecules removed is limited by the size of the pore of the filter, which is disadvantageous in thrombotic thrombocytopenic purpura (TTP), where the ultralarge vWF multimers can be very big. Prior to embarking on plasma exchange therapy, a physician trained in therapeutic apheresis should draw up a treatment plan for each patient [4, 5]. This should include number of plasma volumes to be exchanged during each procedure, type of replacement fluid to be used, frequency and total number of procedures and type of vascular access most suitable for the patient. Also, the potential impact of both the anticoagulation used and the extracorporeal circulatory volume on the patient must be assessed. An exchange of a single plasma volume of the patient is expected to reduce the intravascular concentration of a substance by 60%, and exchanging 1.5 plasma volumes will lower the concentration by 75%; however, further increasing of exchange volume is associated with progressively exchanging more infused replacement fluid and is of diminishing clinical value (Figure 16.1). An exchange of 1–1.5 plasma volumes will suit most clinical situations. Plasma volume in the average adult can be calculated using the following formula:
Plasma Exchange
Introduction
Technologies
Treatment plan
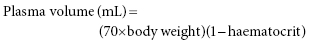
Related posts:
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree