Lactotroph adenomas (prolactinomas) account for approximately 30–40% of all clinically recognized pituitary adenomas. Non-functioning pituitary adenomas account for 25–30% and are the most common pituitary macroadenoma. Non-functioning pituitary adenomas affect males and females equally and usually present in patients over the age of 50 years.
Aetiology
The mechanism of pituitary tumorigenesis is largely unexplained. Pituitary adenomas are monoclonal in origin and are true neoplasms. Mutations in the following genes may play a role in the development of pituitary adenomas:
- Activating mutations of the alpha subunit of the stimulatory G protein (which links the somatotroph cell membrane GH-releasing hormone receptor to adenylate cyclase) are seen in up to 40% of somatotroph adenomas.
- PTTG (the pituitary tumour-transforming gene) is overexpressed in most pituitary adenomas.
- A truncated form of the receptor for fibroblast growth factor-4 has been identified in some pituitary adenomas.
- Loss of function mutations of MEN1 (a tumour suppressor gene) are responsible for tumours that occur in multiple endocrine neoplasia type 1 syndrome.
Physiological and pathological hyperplasia of the pituitary gland should be distinguished from pituitary adenoma. Examples include lactotroph hyperplasia in pregnancy and lactation, thyrotroph hyperplasia in primary hypothyroidism, gonadotroph hyperplasia in primary hypogonadism, and somatotroph and corticotroph hyperplasia due to ectopic GH-releasing hormone and corticotrophin-releasing hormone hypersecretion respectively.
Clinical presentations
The onset of symptoms and signs of pituitary tumours is often insidious, particularly with non-functioning adenomas. Patients tend to present late, and there is usually a delay in diagnosis. Patients with pituitary tumours may present with features of:
- hypersecretion of pituitary hormone(s) in functioning adenomas
- hypopituitarism
- headache
- compression of the surrounding structures such as the optic chiasm.
Hypersecretion of pituitary hormones
- Prolactin-secreting adenomas may present with galactorrhoea, oligo/amenorrhoea, infertility and impotence.
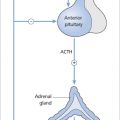
- ACTH-secreting adenomas cause Cushing’s disease (see Chapter 16). ACTH-expressing tumours may also be non-functioning.
- GH-secreting adenomas cause acromegaly (see Chapter 15).
- Luteinizing hormone (LH) and follicle–stimulating hormone (FSH) secretion in LH and/or FSH synthesizing tumours is uncommon and they are usually non-functioning, but they may rarely present with hypogonadism (due to biologically inactive gonadotrophins).
- TSH-secreting adenomas (TSHomas) present with secondary hyperthyroidism with or without a goitre.
Hypopituitarism
A pituitary tumour (usually non-functioning) in the anterior pituitary may result in a decreased secretion of anterior pituitary hormones due to compression or destruction of the surrounding normal pituitary cells. Involvement of the posterior pituitary resulting in diabetes insipidus is less common. The order in which hormone deficiencies develop is GH first, followed by gonadotrophins, ACTH and finally TSH. Signs and symptoms resulting from various pituitary hormone deficiencies are summarized below.
GH deficiency
GH deficiency may present with fatigue, impaired psychological well-being, reduced energy, muscle strength and exercise capacity, and increased abdominal adiposity (fat mass).
LH and FSH deficiency
- LH and FSH deficiency in males may present with reduced libido, impotence, infertility, loss of body hair, fine perioral wrinkles and flushes.
- LH and FSH deficiency in females may present with oligomenorrhoea, amenorrhoea, infertility, dyspareunia, breast atrophy and flushes.
TSH deficiency
Deficiency may present with fatigue, apathy, muscle weakness, cold intolerance, constipation, weight gain and dry skin.
ACTH deficiency
ACTH deficiency may present with fatigue, weakness, nausea, vomiting, weight loss, hypoglycaemia and loss of pubic and axillary hair in females.
Antidiuretic hormone (vasopressin) deficiency
Antidiuretic hormone (ADH, vasopressin) deficiency may present with polyuria, nocturia and polydipsia.
Headache
Headaches are caused by stretching or invasion of the dura. Large tumours with suprasellar extension may occasionally cause obstructive hydrocephalus.
Compression of surrounding structures
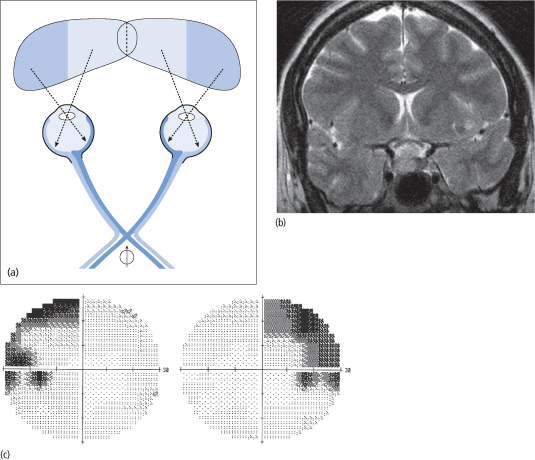
Suprasellar extension of the tumour may result in involvement of the optic chiasm, causing bitemporal visual field loss. Superior quadrants are initially affected, and this gradually progresses to a bitemporal hemianopia that is characteristically asymmetrical (Fig. 12.1). Other rarer presentations include hemi-field slide and post-fixational blindness.
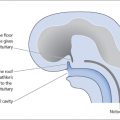
Figure 12.1 (a) Compression of the optic chiasm by a pituitary tumour  affects those nerve fibres that carry the visual impulse from the nasal retina (dark blue), resulting in an inability to view the temporal fields (dark blue areas). (b) Pituitary MRI showing a non-functioning adenoma compressing the optic chiasm. (c) Humphrey visual field revealing bilateral (mostly superior) temporal field defects.
affects those nerve fibres that carry the visual impulse from the nasal retina (dark blue), resulting in an inability to view the temporal fields (dark blue areas). (b) Pituitary MRI showing a non-functioning adenoma compressing the optic chiasm. (c) Humphrey visual field revealing bilateral (mostly superior) temporal field defects.
Stalk compression causes hyperprolactinaemia, which may cause hypogonadism. Other less common complications include third ventricle obstruction (causing hydrocephalus, vomiting, papilloedema, reduced consciousness level) and hypothalamic damage associated with diabetes insipidus and changes in food intake, temperature regulation or behaviour.
Lateral extension and cavernous sinus invasion may cause cranial nerve (IIIrd, IVth, VIth) palsies and diplopia. Inferior extension may result in cerebrospinal fluid (CSF) rhinorrhoea due to erosion of the sphenoid sinus.
Pituitary incidentalomas
Pituitary incidentalomas are mass lesions (usually adenomas) that are detected following radiological imaging of the skull base or brain for another clinical reason.
Management depends on pituitary function and whether the optic chiasm is involved. Microadenomas that are intrasellar and non-functioning may be followed up with an annual magnetic resonance imaging (MRI) scan to monitor their size. Macroadenomas that are non-functioning, have not caused hypopituitarism and do not involve the optic chiasm may also be followed up with an annual MRI.
Investigations
Investigations in patients with suspected pituitary tumours include basal and dynamic pituitary function tests, pituitary MRI and formal visual field assessment. In addition, patients with suspected syndromes of pituitary hormone hypersecretion such as Cushing’s disease and acromegaly should undergo specific investigations for these disorders. These are described in detail in separate chapters.
Basal pituitary function tests
Basal anterior pituitary function tests are summarized in Box 12.2.
Prolactin
Prolactin levels can be elevated in non-functioning adenomas due to stalk compression, resulting in a reduction of the inhibitory effect of dopamine on prolactin secretion (‘disconnection’ hyperprolactinaemia). However, prolactin levels of more than 4000 mU/L are usually due to prolactin hypersecretion from a lactotroph adenoma (prolactinoma).
When measuring prolactin levels, it is important to be aware of two laboratory pitfalls:
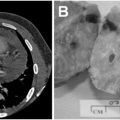
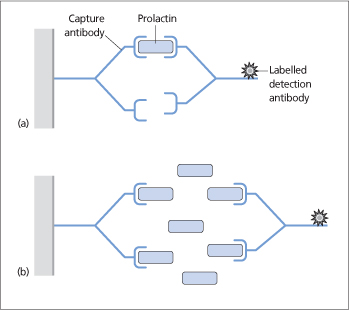
- The ‘hook effect’ occurs when the assay uses antibodies that recognize two different sites on the prolactin molecule. The prolactin molecule is ‘sandwiched’ between these two antibodies: one capturing it and the other labelling it (Fig. 12.2a). Very high prolactin levels may be artefactually reported as normal or only modestly elevated. This is because the very high serum prolactin saturates both the capture and labelling antibodies and prevents the binding of the two in a ‘sandwich’ (Fig. 12.2b). This can be avoided by repeating the assay using a dilution of serum (e.g. 1:100).
- Hyperprolactinaemia may be due to decreased clearance of a complex of prolactin with immunoglobulin G (‘macroprolactin’). This condition is referred to as macroprolactinaemia. Macroprolactin is not biologically active but does get measured in routine assays, thereby causing falsely elevated results. The percentage of macroprolactin should be identified and, if significant, the prolactin result should be amended accordingly. Misdiagnosis can be avoided by pretreating the serum with polyethylene glycol to precipitate the macroprolactin before the immunoassay for prolactin.
Figure 12.2 (a) A two-site immunometric assay for the measurement of serum prolactin uses two antibodies that are specific for different epitopes of prolactin. Prolactin is sandwiched between a ‘capture’ antibody attached to a solid-phase matrix and a labelled detection antibody. (b) The hook effect: very high serum prolactin levels simultaneously saturate both the capture and detection antibodies, preventing sandwich formation and the detection of prolactin.
Insulin-like growth factor-1
Insulin-like growth factor-1 (IGF-1) is a peptide that is synthesized and secreted by the liver under the control of GH. IGF-1 adjusted for sex and age is low in patients with GH deficiency. GH levels are affected by a number of factors including stress, and therefore GH deficiency can only be proven by failure to respond to stimulation in dynamic tests (see below). IGF-1 levels are high in patients with GH-secreting pituitary tumours, causing acromegaly.
LH, FSH, testosterone in men and oestradiol in women
- Women with secondary hypogonadism have reduced oestradiol with reduced or inappropriately normal LH and FSH.
- Men with secondary hypogonadism have reduced testosterone with reduced or inappropriately normal LH and FSH.
TSH and free thyroxine
In secondary hypothyroidism, free thyroxine is low and TSH is either low or inappropriately normal.
8-9 a.m. cortisol and ACTH
In secondary adrenal insufficiency, 8–9 a.m. cortisol levels are low, and ACTH levels are either reduced or inappropriately normal. However, cortisol levels, like GH, are affected by stress, and deficiencies can be proved only by a failure to respond to stimulation in dynamic tests (see below).
ADH (vasopressin) deficiency
Tests of posterior pituitary function include measurement of serum and urine osmolality and sodium concentrations, and the water deprivation test (see Chapter 17).
Dynamic pituitary function tests
Insulin tolerance test
Insulin-induced hypoglycaemia (glucose > 2.2 mmol/L) stimulates GH (to > 20 mU/L or 6 μ g/L) and cortisol (to > 500 nmol/L) in normal individuals. Patients with a basal cortisol below 80 nmol/L are very unlikely to have a normal response and may therefore not need the test.
The insulin tolerance test
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree