Introduction
Pituitary apoplexy is the potentially life-threatening hemorrhage or infarction of the pituitary gland leading to significant neurologic, neuro-ophthalmologic, and endocrine deficiencies. The classic triad of symptoms includes severe headache, vision loss or visual disturbances, and hypopituitarism with or without impaired consciousness that is acute and sudden in onset, but the symptoms can be mild and subacute in nature manifesting over days to even months. Although pituitary apoplexy most commonly arises from an existing pituitary adenoma, it can rarely occur in a normal pituitary gland such as after significant postpartum hemorrhage and acute hypovolemic shock. Here, we review the epidemiology, pathophysiology, clinical presentation, diagnosis, and management of pituitary apoplexy.
Epidemiology
Pituitary apoplexy is rare, with a reported prevalence of 6.2 cases per 100,000 individuals and an incidence of 0.17 episodes per 100,000 per year. Of patients with a known diagnosis of pituitary adenoma, the incidence of pituitary apoplexy ranged from 2% to 14.1% with a higher incidence in macrodaenomas than microadenomas. , , Whereas pituitary adenomas such as prolactinomas are found more frequently in women than in men, pituitary apoplexy is seen in both sexes, with some studies finding a higher incidence in men. Pituitary apoplexy is more frequent in the fifth or sixth decade but can be found at all ages. ,
PRESENCE OF PITUITARY ADENOMA
Excluding incidental postmortem findings of pituitary apoplexy, 93% to 100% of reported pituitary apoplexy cases occur in patients with pituitary adenomas. In rare cases, pituitary apoplexy arises from other pituitary and sellar lesions such as Rathke’s cleft cyst (RCC), , , , craniopharyngioma, sellar tuberculoma, hypophysitis, , , sellar abscess, sellar metastasis, or in normal pituitary gland postpartum, from acute hypovolemic shock (Sheehan’s syndrome). Apoplexy occurs in approximately 10% of existing, known pituitary adenomas, but estimates vary widely, as many pituitary tumors are undetected. , , , Apoplexy is the first presentation of pituitary disease in 73% to 97% of patients. , , In incidentally detected pituitary adenomas, the risk of apoplexy is very low, with an incidence of only 0.2% of patients/year. The incidence increases to 0.83% to 1.1% in larger incidentally found pituitary tumors or macroincidentalomas; however, it may be higher in those with predisposing factors such as anticoagulation therapy or in faster- growing tumors. Of note, subclinical (asymptomatic) pituitary apoplexy is frequent, affecting up to 25% of all pituitary tumors on imaging or at autopsy.
Larger adenomas and nonfunctioning adenomas are most likely to apoplex. Of patients presenting with pituitary apoplexy related to an adenoma, 90% to 100% have adenomas measuring over 1 cm, and 6% to 38% over 2.5 cm. , , , , , , , In most studies, 10% to 24% of tumors are completely necrotic and cannot be typed on pathologic examination. , , , Of tumors that can be typed, 70% to 90% are nonfunctioning pituitary adenomas, most likely because they grow larger than secretory tumors while remaining asymptomatic. , , , , , , Subtypes of nonfunctioning pituitary adenomas include silent gonadotropic hormone-expressing, null-cell (displaying no particular cell lineage differentiation), plurihormonal, silent adrenocorticotropic hormone (ACTH)-expressing, and growth hormone–expressing adenomas. , , After nonfunctioning pituitary adenomas, prolactinomas result in 3% to 16%, growth hormone producing adenomas in 2% to 10%, and ACTH producing adenomas in 0% to 7% of pituitary apoplexy cases. , , , , , , In children and adolescents, pituitary apoplexy is usually associated with prolactinomas rather than nonfunctioning adenomas. ,
Pathophysiology
The pituitary gland is particularly prone to both bleeding and ischemic infarction, with a higher rate of spontaneous infarction than other central nervous system tumors. Pituitary tumors require a significant amount of energy, are particularly sensitive to glucose deprivation, and contain few, fragile blood vessels compared with other tumors. Pituitary tumors also tend to have high intratumoral pressure. Patients with small sellas may be more prone to pituitary apoplexy, likely because pressure on a pituitary tumor decreases blood flow and may precipitate apoplexy. , When a pituitary tumor infarcts or hemorrhages, it often leads to extensive coagulation necrosis of large parts of the normal pituitary gland. The underlying molecular events leading to pituitary apoplexy are unclear and may include vascular endothelial growth factor, matrix metalloproteinases, hypoxia-inducing factor-1α, and inflammatory cytokines, among others; however, well-designed genomics, proteomic, and metabolomics studies using large sample sizes are lacking and are essential for the elucidation of the underlying molecular mechanisms of pituitary apoplexy.
Precipitating Factors
Identifiable factors precipitate pituitary apoplexy in 15% to 40% of cases. , , , These potential precipitating factors can be broadly placed into two categories: (1) vascular causes such as blood pressure fluctuations and increased bleeding risk; and (2) increased pituitary demand such as during pregnancy, hormonal treatment, and pituitary testing. Additional precipitating factors may include radiation therapy and infiltrates like lymphocytic hypophysitis. However, for the majority of pituitary apoplexy cases, there is no clear underlying precipitant.
VASCULAR FACTORS: BLOOD PRESSURE FLUCTUATIONS AND BLEEDING RISK
Pituitary apoplexy often results from vascular factors such as (1) an acute decrease in perfusion of the pituitary leading to infarction, or (2) an increase in bleeding tendency or an acute increase in blood pressure leading to hemorrhage.
Any procedure that causes hemodilution, hypotension, or microemboli can potentially cause infarction of pituitary tissue, including surgical, angiographic, intravascular, and endoscopic procedures. , , , , In a 2019 single-center retrospective study, 5% of patients had extracranial surgery within the past 24 hours before the pituitary apoplexy. Cardiopulmonary bypass dynamics during cardiac surgery has been reported to cause infarction of the pituitary. , , In one case report in a patient with atherosclerosis of the internal carotid arteries, lumbar fusion surgery in the prone position led to increased intraabdominal pressure and compression of the vena cava, lowering cardiac index, in addition to compressing the vertebral arteries, all leading to decreased perfusion to the pituitary, precipitating pituitary infarction. Episodic hypotension from a variety of etiologies, including severe sepsis, hemodialysis, and phosphodiesterase type 5 (PDE5) inhibitors (e.g., vardenafil), may lead to pituitary apoplexy. , , In one reported case, cardiac pharmacologic nuclear stress testing with regadenoson precipitated apoplexy, likely due to cerebral vasodilation and blood pressure fluctuation.
Large, transient increases in blood pressure can precipitate pituitary hemorrhage. In one retrospective observational study of surgical cases, 2 out of 32 patients who presented with pituitary apoplexy were intoxicated with methamphetamine, possibly leading to acutely elevated blood pressure. It is unclear if chronic hypertension also predisposes to pituitary apoplexy. A preceding diagnosis or acute presentation of hypertension (systolic BP greater than 140 mm Hg) is present in 5% to 48% of patients with pituitary apoplexy. , , , , , , , ,
Anticoagulation and thrombocytopenia are risk factors for hemorrhage, including in the pituitary gland. Between 5% and 25% of patients with pituitary apoplexy are on antiplatelet agents or anticoagulation. Use of aspirin, dabigatran, apixaban, rivaroxaban, warfarin, and heparin have all been implicated in pituitary apoplexy. , , , , , , , , However, due to a lack of randomized studies, it is difficult to give evidence-based recommendations regarding the use of antiplatelet and anticoagulation agents in individuals with known pituitary adenomas. Head trauma, especially in patients on antiplatelet or anticoagulation therapy, can precipitate bleeding of pituitary tumors. , , Cytotoxic chemotherapy resulting in thrombocytopenia may also predispose to pituitary apoplexy. ,
INCREASED DEMAND: PREGNANCY, PITUITARY GLAND TESTING, AND HORMONAL THERAPY
Increases in pituitary demand, such as during pregnancy, pharmacologic stimulation, or pharmacologic treatment, can exceed available blood supply and lead to infarction of the pituitary. Although this is most common in patients with pituitary adenomas, it can happen in a normal pituitary under special circumstances like pregnancy.
Pituitary demand increases in pregnancy, predisposing to infarction. Of women who present with pituitary apoplexy, 8% to 25% are pregnant or postpartum. , , During pregnancy, placental estrogen stimulates lactotroph cells to secrete prolactin, resulting in lactotroph hyperplasia. This leads to increases in pituitary size, and therefore increases intracapsular pressure, compressing the blood supply to the pituitary, leaving it vulnerable to hypotension, while simultaneously increasing pituitary demand. The pituitaries of patients with small sellas are especially compressed. The increased metabolic demand combined with decreased blood supply can result in pituitary apoplexy. , , In one study of women who presented with pituitary apoplexy in the third trimester, half had pre-eclampsia, indicating that hypertension or blood pressure fluctuations might also play a role. In Sheehan’s syndrome, severe postpartum hemorrhage leads to hypotension and infarction of the pituitary. Postpartum-disseminated intravascular coagulation can also lead to necrosis of the pituitary.
Pharmacologic stimulation of the pituitary gland increases metabolic demand of the pituitary gland, and, especially in patients with existing pituitary tumors, can precipitate pituitary apoplexy. Inferior petrosal sinus sampling, in which ACTH is measured in petrosal and peripheral venous plasma from catheters inserted into both inferior petrosal veins before and within 10 minutes after administration of corticotropin-releasing hormone, can cause pituitary apoplexy in vulnerable patients. Recent guidelines recommend against gonadotropin-releasing hormone (GnRH) or thyrotropin-releasing hormone stimulation tests given the rare but serious risk of apoplexy.
Pituitary apoplexy has also been reported as a complication of hormonal treatments including use of the GnRH agonist leuprolide acetate for both prostate carcinoma and for fertility treatment, and with use of the dopamine agonist cabergoline for prolactinoma in children and adults. , , , Unfortunately, there are no specific guidelines or biomarkers to help determine which patients with prolactinomas are at increased risk for developing pituitary apoplexy with dopamine agonist treatment. Additionally, at least two cases have been reported of children with prolactinomas who developed pituitary apoplexy 2 weeks after starting growth hormone.
Clinical Presentation
As initially described by Brougham et al., the classic presentation of pituitary apoplexy is the sudden onset of impaired consciousness, headache and stiff neck, ocular palsies, and sometimes amblyopia or hemiparesis ; however, the actual neurologic signs and symptoms can vary widely ( Table 21.1 ). , , , , , Additionally, a majority of patients with pituitary apoplexy will have significant endocrine dysfunction that could be life-threatening. Although the classic presentation of pituitary apoplexy is acute, symptoms may present in a subacute or chronic fashion with a range of hours to months from initial onset to progression of symptoms. ,
| Symptom | Incidence (%) |
|---|---|
| Headaches | 83–100 |
| Visual acuity loss | 23–55 |
| Hypopituitarism | 58–88 |
| Visual field loss | 22–63 |
| Nausea | 29–59 |
| Ocular palsies | 25–79 |
| Altered level of consciousness/coma | 3–19 |
HEADACHE AND IMPAIRED CONSCIOUSNESS
Headache due to meningeal irritation is the most prominent and common manifestation of pituitary apoplexy, reported to occur in 83% to 100% of cases. , , , , , The most classic presentation of the headache is severe and acute, often described as a “thunderclap” and easily confused with the headache of a subarachnoid hemorrhage, but the headache may have a more insidious, subacute onset. , , , There is no classic distribution for the headache: unilateral, frontal, temporal, occipital, and apical headaches have all been described. , Other signs and symptoms of meningeal irritation, including nuchal rigidity and photophobia, are often reported alongside the headache, , , and 29% to 59% of patients report nausea and vomiting. , , ,
In the classic paper by Brougham et al., altered mental status was the most frequent neurologic abnormality found in pituitary apoplexy ; however, recent reports have found an overall lower incidence. Between 3% and 19% of patients present with altered level of consciousness ranging from lethargy to stupor and even coma. , , , In a review of patients with pituitary apoplexy during pregnancy and postpartum, 8% presented with altered mental status. Altered mental status due to pituitary apoplexy can be subtle and masquerade as functional decline in older adults, especially in subacute presentations.
VISUAL DISTURBANCES AND OTHER FOCAL NEUROLOGIC DEFICITS
Focal neurologic and neuro-ophthalmologic deficits occur in pituitary apoplexy from compression of cranial nerves and blood vessels. Visual disturbances, presenting acutely or progressively, are very common: 23% to 55% of patients have a visual acuity deficit and 22% to 63% have a visual field deficit. , , , , Visual disturbances correlate with larger tumors. Rarely, pituitary apoplexy presents with bilateral blindness, possibly accompanied by fixed dilated pupils and optic atrophy. ,
Ocular palsies are present in 25% to 79% of patients, with involvement of oculomotor nerve palsy being most frequent resulting in limited adduction with exotropia and hypotropia, ptosis, and mydriasis. , , , , , , Anisocoria or abnormal pupil responses occur in about 20% to 27% of cases. , Ocular palsies are correlated with larger tumors, panhypopituitarism, and tumor necrosis.
Rarely, pituitary apoplexy can lead to compression of one or both internal carotid arteries, which can lead to infarction of large cerebral arterial territories or even bilateral cerebral hemispheres without early intervention. , , Additionally, cerebral infarction secondary to arterial vasospasm has also been reported and should not be overlooked as a possible complication of pituitary apoplexy. ,
ENDOCRINE DYSFUNCTION
On presentation with pituitary apoplexy, 58% to 88% of patients have some form of endocrine deficiency, often silent but sometimes leading to fatigue, menstrual disturbances, and reduced libido. , , , , , , , Cortisol deficiency is common, affecting 23% to 86% of all patients. , , , , , Cortisol deficiency leads to lack of suppression of ADH release, which results in hyponatremia (serum sodium less than 135 mmol/L) in 3% to 47% of patients. , , In severe cases, patients can present with acute adrenal insufficiency, possibly leading to significant hyponatremia, hypotension, and loss of consciousness, requiring immediate empiric steroid replacement. , , , Other hormonal abnormalities are also common. From recent literature, 30% to 69% of patients present with hypogonadism, , , , , , , 26% to 83% with hypothyroidism, , , , , , , and 11% to 32% with growth hormone deficiency. , , , , Occasionally, patients present with diabetes insipidus (1% to 3%) , , , or low serum prolactin. , Of note, the reported studies include only surgically managed patients, who tend to present with more severe deficits.
Diagnosis
The diagnosis of pituitary apoplexy is made by clinical assessment and confirmed by radiologic detection of the pituitary apoplexy or pituitary tumor, even if no necrosis or hemorrhage is found. Because pituitary apoplexy can present with headache and meningeal signs, the differential diagnosis often includes subarachnoid hemorrhage and bacterial meningitis. Less often, if the patient suffers from cerebral ischemia, then pituitary apoplexy may be mistaken for ischemic stroke or, if the patient presents with hypotension or other hemodynamic instability, for myocardial infarction. Diagnosis in pregnancy can be challenging. Lactotroph hyperplasia may lead to sudden-onset headache, visual changes, and hypopituitarism without apoplexy. In that case, magnetic resonance imaging (MRI) without contrast will show homogenous signal characteristics consistent with an enlarged pituitary, but will not show the ischemia or hemorrhage typical of pituitary apoplexy. ,
CLINICAL ASSESSMENT
Lumbar puncture has limited utility in differentiating pituitary apoplexy from subarachnoid hemorrhage, as both will cause cerebrospinal fluid to have increased cerebrospinal fluid leukocytes, erythrocytes, and protein. Lumbar puncture in pituitary apoplexy may also show aseptic meningitis. However, lumbar puncture is essential if bacterial meningitis is suspected, in which case cerebrospinal fluid cultures should be obtained. ,
Endocrine testing can also help differentiate pituitary apoplexy from other causes of headache and meningismus. Although a comprehensive hormone assessment will be important in guiding management of the endocrine dysfunction, it should not delay treatment, particularly with steroid therapy for presumed acute secondary adrenal insufficiency.
RADIOLOGIC IDENTIFICATION
In order to confirm the diagnosis, neuroimaging is used to detect pituitary apoplexy or the underlying pituitary tumor by computed tomography (CT) or MRI. Imaging of the pituitary gland can reveal a simple infarction, hemorrhagic infarction, mixed hemorrhagic infarction and clot, or pure clot with mixed features commonly seen.
Computed Tomography
CT is fast and widely available but nonspecific. Acute hemorrhagic infarct of the pituitary gland may be seen on CT as a large heterogeneously hyperintense sellar mass, but aneurysms, meningiomas, RCC, germinomas, and lymphomas may also present similarly. , MRI is more sensitive for acute pituitary hemorrhage, and CT will fail to detect most nonhemorrhagic infarcts. , CT will, however, usually identify a pituitary or suprasellar mass. In one study, 60% of patients were initially diagnosed on CT before a subsequent dedicated pituitary MRI. In another, CT was very effective in showing a pituitary mass but could only identify hemorrhage in 40% of cases, whereas MRI identified 89% of hemorrhages. When a rapid diagnosis is needed, CT without contrast is most useful in the emergency setting to exclude a large bleed such as a subarachnoid hemorrhage or a large mass lesion before a dedicated pituitary MRI is obtained.
Magnetic Resonance Imaging
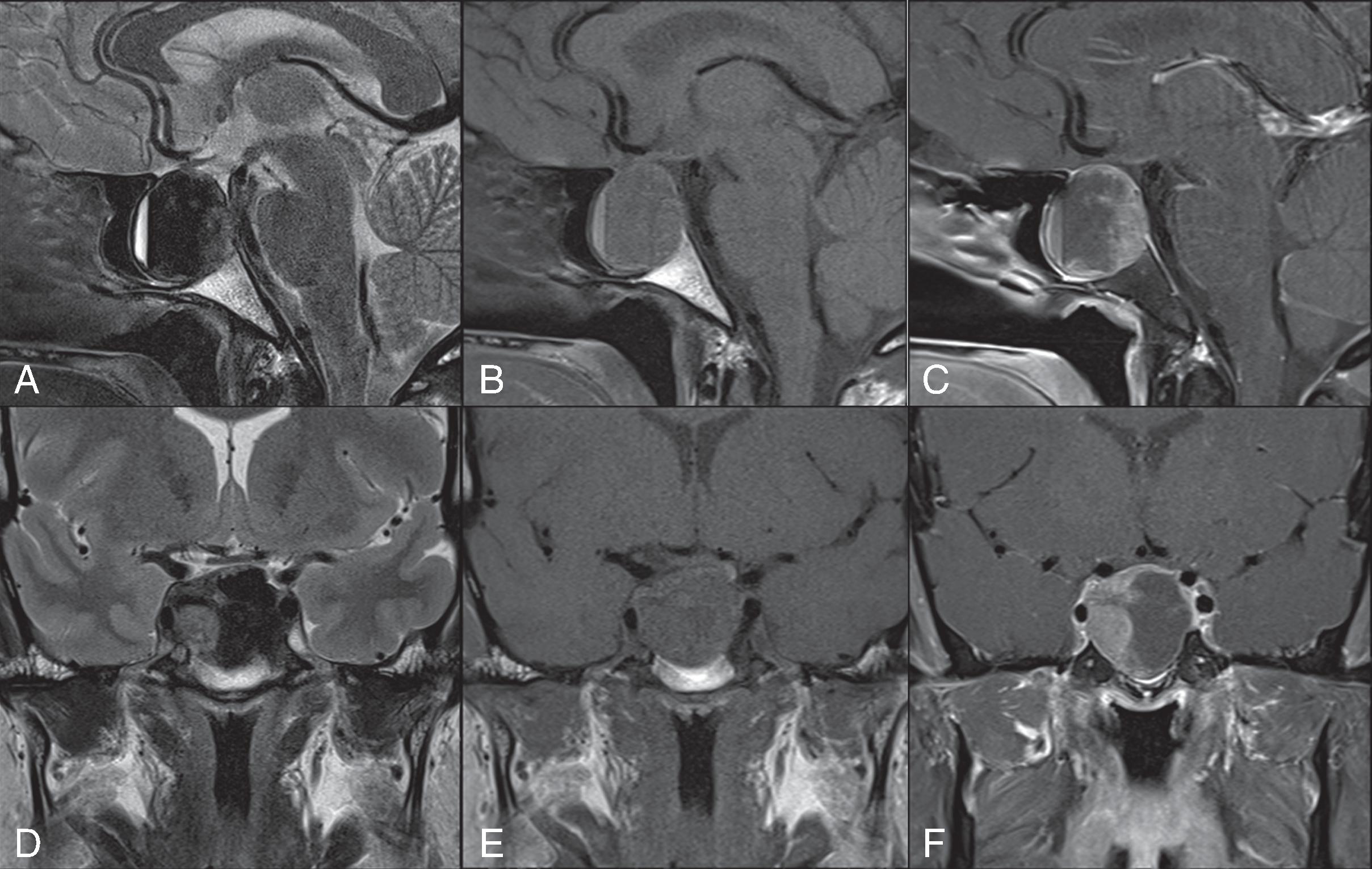
The American College of Radiology (ACR) recommends MRI without contrast using a high-resolution pituitary protocol as the optimal first-line imaging test in pituitary apoplexy. MRI without contrast is highly sensitive for both hemorrhage and infarction. MRI can show tumor enlargement, sellar expansion, and intratumoral hemorrhage, which will appear as a T1 signal hyperintensity, a low T2 signal, or a hemorrhage fluid level ( Fig. 21.1 ). The radiologic differential diagnosis on MRI includes metastasis to the pituitary, pituitary abscess, craniopharyngioma, RCC, and sellar aneurysm, all of which may also present with headache and visual changes. , The ACR recommends including T1 fat saturation sequences in order to differentiate hemorrhage from fat in other soft-tissue masses such as craniopharyngiomas, RCC, dermoids, and teratomas. During pregnancy, diagnosis of pituitary apoplexy on MRI can be especially difficult, because the pituitary gland enlarges and can appear similar to a pituitary adenoma, lymphocytic hypophysitis, and pituitary apoplexy. MRI with IV contrast may be performed for use in operative guidance but is usually not necessary for diagnosis.