html xmlns=”http://www.w3.org/1999/xhtml”>
CHAPTER 7
Pipeline Diabetes Therapies
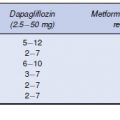
The complexity of glucose metabolism and the number of cellular processes affected by diabetes provide ample opportunity for the development of new drug targets and first-in-class molecules (Table 7.1).
Taspoglutide
Taspoglutide is a glucagon-like peptide 1 (GLP-1) receptor agonist with 93% homology to endogenous GLP-1 and is currently in Phase 3 trials. When administered as a once-weekly subcutaneous injection to people with type 2 diabetes, plasma concentrations of the drug peak within 24 hours after injection and are associated with significant reductions in fasting and postprandial plasma glucose compared with placebo for up to 14 days after the initial injection (Kapitza et al., 2009). In an eight-week, dose-ranging study in patients with type 2 diabetes inadequately controlled with metformin alone, all doses of taspoglutide achieved significantly greater reductions in HbA1c compared with patients receiving placebo plus metformin (Nauck et al., 2009). The mean decrease in HbA1c of 1.1% from a baseline of 7.9 compares favourably with available GLP-1-receptor agonists. Taspoglutide also produced a progressive and dose-dependent weight loss. Similar to other GLP-1 receptor agonists, the most frequent adverse event with taspoglutide treatment was mild-to-moderate nausea. Unfortunately, further development of this agent has been (temporarily?) suspended because of hypersensitivity reactions in some patients.
Linagliptin
Linagliptin is a dipeptidyl peptidase-4 (DPP-4) inhibitor in Phase 3 development for the treatment of type 2 diabetes with a long duration of action making it suitable for once-daily dosing (Heise et al., 2009). The Phase 3 programme with linagliptin comprises five pivotal trials, which are evaluating the efficacy and safety of linagliptin alone and in combination with commonly used diabetes treatments including metformin, sulphonylureas and thiazolidinediones. Data from a 12-week Phase 2 study in 333 patients failing to achieve glycaemic control despite being treated with metformin, showed statistically significant reductions in HbA1c, with placebo-corrected reductions from baseline of 0.73% and 0.67% for linagliptin 5 mg and 10 mg, respectively (Forst et al., 2010). In this study, the incidence of adverse events was similar to placebo. At the time of writing, data from only one of the pivotal Phase 3 trials have been published. In this trial, 700 patients inadequately controlled on a maximum tolerated dose of metformin monotherapy were randomized to linagliptin 5 mg (n = 523) or placebo (n = 177) (Taskinen et al., 2011). After 24 weeks of treatment, the difference in mean change from baseline HbA1c was –0.64% compared with placebo (P < 0.0001). Linagliptin was also associated with significantly greater reductions in both fasting and postprandial plasma glucose. The drug had no influence on body weight and was well tolerated. In contrast to other DPP-4 inhibitors, only a minor fraction of linagliptin is eliminated through the kidneys, which may be an advantage in patients with renal impairment.
Table 7.1 Pipeline therapies for type 2 diabetes
| Pipeline therapy | Mechanism of action | Stage of development |
| Taspoglutide | GLP-1 receptor agonist | Phase 3 |
| Linagliptin | DPP-4 inhibitor | Phase 3 |
| Bile acid receptor agonists | Activate TGR-5 to increase energy expenditure and secretion of GLP-1 | Phase 2 |
| Glucokinase activators | Increase sensitivity of glucokinase to glucose promoting insulin secretion and increasing hepatic glucose uptake | Phase 2 |
| Sirtuins | Stimulate mitochondrial activity in metabolically active tissues | Phase 2 |
| Sodium-glucose cotransporter-1 inhibitors | Decrease intestinal glucose absorption Phase 1 | Phase 1 |
| Sodium-glucose cotransporter-2 antisense inhibitors | Inhibit expression of the SGLT-2 gene | Phase 1 |