Atmospheric Zones
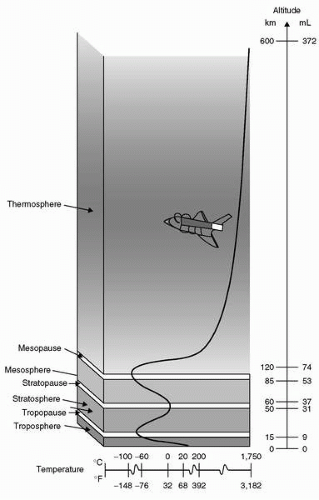
Temperature and its variation provide much of the basis for subdivisions of Earth’s atmosphere into regions defined in
Figure 3-1. The lowest zone, the
troposphere, is the only region of Earth’s atmosphere capable of supporting human habitation without artificial support. The troposphere starts at the Earth’s surface and extends to the
tropopause, between 5 and 9 mi [8 to 14.5 kilometer (
km); 26,000-48,000 ft]. At its higher levels, above 20,000 ft (3.8 mi; 6
km), at least some degree of artificial support is required in the form of supplemental oxygen. A linear decrease in temperature characterizes the troposphere from sea level (15°C) to the tropopause, typically at approximately 35,000 ft (10.7
km), where the temperature is approximately −55°C. The lapse rate, that is, the rate of decreasing temperature with increase in altitude in the troposphere, is −2°C or approximately −3.5 F per 1,000 ft. Approximately 80% of the atmospheric mass and most of the weather phenomena occur in the troposphere. Variations in temperature, pressure, and humidity in the troposphere account for extreme differences in the environmental conditions we experience as weather.
The tropopause is the division between the troposphere and stratosphere. Aircraft jet engines perform with greater efficiency at lower temperatures, which is one reason cruise is planned near the tropopause where the temperature is lowest. The
stratosphere starts just above the tropopause and extends up to 50
km (31 mi). Ninety-nine percent of the mass of the air is located in the troposphere and stratosphere. The temperature throughout the lower part of stratosphere is relatively constant. Compared to the troposphere, this part of the atmosphere is dry and less dense. The temperature in this region increases gradually to −3°C due to the absorption of ultraviolet (UV) radiation. This radiation reaching the lower stratosphere from the sun is responsible for creation of ozone, the ozone layer, or ozonosphere. In the process of ozone production and in reactions with ozone, nearly all of the UV radiation is absorbed including the most hazardous form to life, UV-C (wavelengths <280 nm). Much of UV-B (wavelengths between 280 and 320 nm) is also absorbed, although the UV-B reaching the surface is sufficient to be a major cause of melanoma cancers and sunburn. Most of the UV-A (wavelengths between 320 and 400 nm) reaches the Earth’s surface, but is needed by humans for production of vitamin D. Although flight in the upper troposphere and lower stratosphere involves exposure to more UV radiation than on the surface, no health risk is currently associated with routine flying operations (see
Chapter 8). Flight above the stratosphere and space flight involve risk of exposure to significant levels of radiation.
The higher regions of the atmosphere, 50,000 ft and above, are so thin that pressure suits are required to sustain life. Temperature variations result from variable absorption of the sun’s energy in several forms and thermal protection must be incorporated for any exposure in these regions. In the higher regions, flight of air-breathing aircraft becomes impossible and control surfaces are no longer effective. Further description will be left to the references and recommended reading.
The subdivision of the zones described in the preceding text relates to the ability of humans to function based on the partial pressure of oxygen available and need for artificial pressure to sustain life (
Table 3-2).
Altitude
Altitude is measured in many different ways using different standards for different purposes. On low-altitude maps provided to pilots, the height of physical features of Earth, like mountains and airfields, is measured in feet above mean sea level (MSL). MSL is the average height of the surface of the sea for all stages of the tide over a 19-year period, usually determined from hourly height readings. With properly set, calibrated, and functioning altimeters, feet above MSL is the altitude viewed by the pilot in an aircraft. This is also known as
pressure altitude (PA), the altitude in the Earth’s atmosphere above the standard datum plane, standard sea level pressure, measured by a pressure altimeter. Pilots are quite interested in the height of their aircraft above the ground. This altitude, above ground level (AGL), is determined by subtracting the elevation in feet above MSL of the ground below the aircraft from the elevation of the aircraft. The routine determination of a safe altitude on a route between navigational aids to avoid terrain and towers is usually viewed on low-level navigational maps as the minimum en route altitude (MEA), which is the altitude between radio fixes that assures acceptable navigational signal coverage and meets obstruction clearance requirements between those fixes. Flying at that altitude with a properly set altimeter ensures adequate separation from obstacles for the entire route segment. PA is the height in the atmosphere at which a given value of standard pressure exists. With 29.92 in. of Hg set in the Kollsman window of the
altimeter, PA is displayed in feet on the altimeters of United States Air Force (
USAF) aircraft. Because hectoPascals are a standard in parts of the world, some confusion can arise when pilots “assume” that, for example, 988 means 29.98 in. of Hg when given by an air traffic controller as an altimeter setting because some controllers in the United States leave off the “2.”
Air Density, Pressure, and Temperature
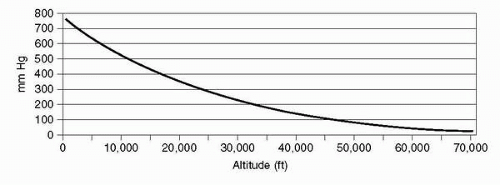
The density of air is affected by its pressure, which decreases exponentially with increasing altitude, reaching 50% of sea level density and pressure at approximately 18,000 ft (5.49
km). This relationship is affected in any specific locale by deviations from standard temperature and pressure.
Figure 3-2 graphically shows how atmospheric pressure is affected by altitude. The curve depicts how each 10,000-ft increase in altitude results in less change in pressure; 0 to 10,000 ft changing by 237 mmHg, 10,000 to 20,000 ft changing by 173 mmHg, and 40,000 to 50,000 ft changing by only 54
mm Hg.
During takeoff, landing, and low-level phases of flight, aircraft altimeters are routinely set to the field altimeter setting to account for variations in local pressure. This procedure avoids significant errors in altitude of the airfield versus what is indicated on the altimeter. Temperature variations from the standard temperature of 15°C also produce errors, which affect terrain clearance. For instance, an aircraft flying at 5,000 ft in −40°C (e.g., Alaska in the winter) would be more than 1,200 ft lower than the indicated altitude
after correction for local barometric pressure (P
B). Local P
B in the United States is based on inches of Hg. This setting would show the altitude of 0 ft at sea level on such a day. As the local pressure varies, altimeters are set to higher or lower settings to yield the correct field elevation on an aircraft altimeter at a designated point on that field. Above 18,000 ft (flight level 180; altitude in ft/100), altimeters
are routinely set to 29.92 in. of Hg to provide adequate and standardized clearance for aircraft altitude separation. Although the inches of Hg standard for altimeter settings are a pressure indication, it is not normally used in aviation for describing total atmospheric pressure at a given altitude. Elevation is typically measured in ft, meters
(m), or
km and pressure in psia,
mm Hg, or mb.
The Gas Laws
A basic understanding of the gas laws is necessary to comprehend the physical nature of the atmosphere and how it interacts with human physiology. The gas laws define physical properties of our atmosphere and provide a basis for understanding how they affect our function during exposure to reduced atmospheric pressure.
Boyle’s Law
Robert Boyle (1627-1691) was an Anglo-Irish scientist noted for his work in physics and chemistry. In 1662, Boyle published the finding which states that at a constant temperature, the volume of gas is inversely proportional to its pressure. P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume. Solving this equation for the volume of a contained gas at a different pressure quantitatively describes trapped gas expansion with reduced pressure.
P1 × V1 = P2 × V2 or P1/P2 = V2/V1
Solving this equation to find the volume of a liter of dry gas taken from sea level to 20,000 ft and 40,000 ft, assuming unrestricted expansion, would result in the following:
1.0 L at sea level
(760 mmHg × 1 L)/349 mmHg = 2.2 L at 20,000 ft
(760 mmHg × 1 L)/141 mmHg = 5.4 L at 40,000 ft
The problem becomes more complicated by the inclusion of water vapor in the lungs and other spaces in the body as
described in the section Trapped Gas, Section 3.
Figure 3-4 shows the volume and diameter of a wet gas sphere at various pressures and graphically shows how Boyle’s law works on trapped gases during decompression and recompression, descent.
Dalton’s Law
John Dalton (1766-1844) was an English chemist and physicist. In 1803, he observed that the total pressure of a mixture of gases is equal to the sum of the partial pressures of each gas in the mixture.
PT = P1 + P2 + P3 + … Pn
Because the standard atmosphere at sea level is 760
mm Hg, Dalton’s law indicates that the sum of partial pressures of the gases that make up the standard atmosphere must equal 760
mm Hg. The pressure of each gas in a mixture of gases is independent of the pressure of the other gases in that mixture. Multiplying the percentage of a gas in the mixture times the total pressure of the mixture yields the partial pressure of that gas.
The standard atmosphere does not include water vapor pressure, primarily due to its variation in the Earth’s atmosphere between 0% and 100% relative humidity. This variation amounts to 0% to 6.2% of 760 mmHg, or 0 to 47
mm Hg at body temperature, 37°C.
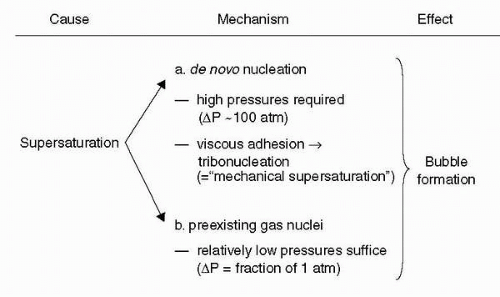
Henry’s Law
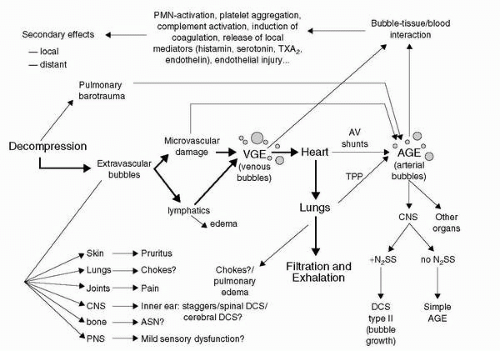
William Henry (1775-1836) was an English chemist who, in 1803, published his findings that the amount of a gas in a solution varies directly with the partial pressure of that gas over the solution. This relationship explains why dissolved nitrogen transitions to a gas phase in blood and tissues during decompressions sufficient to result in supersaturation. The resulting bubbles of nitrogen with minor amounts of oxygen, carbon dioxide, and water vapor can cause decompression sickness (
DCS).
Charles and Gay-Lussac’s Law
Jacques Alexandre César Charles (1746-1823) was a French inventor, scientist, mathematician, and balloonist. In 1783, he made the first balloon using hydrogen gas; upon release, it ascended to a height of approximately 3
km (2 mi). In 1787, he discovered the relationship between the volume of gas and temperature, known variously as
Gay-Lussac’s law or
Charles’s law.V1/V2 = T1/T2 or V1/T1 = V2/T2
Charles did not publish his findings and Joseph Louis Gay-Lussac first published the finding in 1802, referencing Charles’ work. The temperature is in Kelvin degrees, where °K = °C + 273. At absolute zero, −273°C, the Kelvin temperature is 0°K. The distinction between Boyle’s law and Charles’ law is what is held constant, whereas the other two parameters are varied. Boyle’s law describes changes in volume with respect to pressure when temperature is held constant. Charles’ law describes how volume changes with temperature when pressure is held constant. Although Charles’ law is very important from an engineering and chemistry standpoint, the temperature of human body is usually rather constant at body temperature, limiting its effect on physiology. Changes in all three parameters (volume, pressure, and temperature) are better described by the Ideal Gas law, which includes the three parameters in one equation with other factors to improve accuracy.
PV = nRT
where
P = pressure,
V = volume, T = temperature, n = number of moles, and R = universal gas constant = 8.3145 J/mol K.
Historical Aspects
Sir Robert Boyle did pioneering work in the field of high-altitude medicine and was the first to observe bubble formation in vivo in one of his experimental animals during decompression in a hypobaric chamber
“I shall add on this occasion… what may seem somewhat strange, what I once observed in a Viper… in our Exhausted Receiver, namely that it had manifestly a conspicuous Bubble moving to and fro in the waterish humour of one of its Eyes.”
Subsequent clinical evidence of
DCS in humans came from air-pressurized mineshaft operations. M. Triger, a French mining engineer, reported in 1841 pain and muscle cramps in coal miners (
26). In 1854, two French physicians, B. Pol and T. J. J. Watelle, gave an account of the circumstances in which the disease develops upon exiting the compressed air environment: “One pays only on leaving” and recognized as well that recompression ameliorated the symptoms. They were the first to use the term
caisson disease named for the compressed air environment the workers were exposed to—analogous to the diving bells (caissons) (
27).
In 1869, the French physician L. R. de Mericourt published the first comprehensive medical report on
DCS in divers (
27). The French physiologist Paul Bert described in his classic treatise
La pression barométrique (1878) the relationship between bubbles and symptoms of
DCS during rapid decompression (
28).
The advent of balloons and aircraft with sufficient performance to attain significant altitudes brought the clinical syndrome into the realm of aerospace medicine. In 1906, H. Von Schrötter described in his book
Der Sauerstoff in der Prophylaxe und Therapie der Luftdruckerkrankungen the symptoms he experienced in a steel chamber after ascending in 15 minutes to 8,994 m (29,500 ft) closely resembling caisson disease (
29). Von Schroetter discounted that hypothesis, but Boycott, Damant, and Haldane reviewed his account and wrote in an article in 1908 (
30):
“Although he concludes that these symptoms could not have been due to caisson disease, we think in view of the data given by Damant and ourselves, that he was probably mistaken, and that the risk of caisson disease at very low pressure ought to be taken into account.”
This is the first clear reference to altitude
DCS in the literature. In 1917, Professor Yandell Henderson provided a detailed theory in which he postulated that it would be possible to get
DCS from altitude exposure (
31).
J. Jongbloed described in his thesis in 1929 (
32,
33) the effects of simulated altitude on human subjects and called attention to the similarities of compressed air illness and
DCS of altitude. In 1931, Barcroft et al. (
34) described pain in the knees experienced in the hypobaric chamber while exercising at altitudes of 9,160 m (30,000 ft), which in hindsight were
most likely manifestations of
DCS. In the United States, Dr. H. Armstrong researched the effects of decreased
PB on the aviator and described in 1939 bubble formation that he experienced himself while at altitude in the hypobaric chamber (
35):
“… Then I noticed a series of small bubbles in the tendons of my fingers … I was certain in my mind they represented aeroembolism..”
In 1938, Boothby and Lovelace reported a case of transient paraplegia in a fellow physiologist (Dr. J. W. Heim) during an ascent to 10,670 m (35,000 ft) while on oxygen; the paraplegia disappeared upon repressurization to ground level. This case illustrated the potential for serious neurologic
DCS at altitude and spurred more research (
36).
The recent decades of research have introduced new monitoring capabilities, which have allowed investigation of the bubble manifestation of the disease under controlled laboratory conditions. Ultrasonic echo-imaging Doppler measurements as an index for gas evolution have enhanced our capability to investigate the
in vivo venous gas phase. The degree of venous bubbles present is graded on a numerical scale referred to as a
venous gas emboli (VGE) score. The first such scoring system was devised by Spencer in 1976 (
37); on this 0 to 4 scale a score of 0 refers to no bubbles and a score of 4 refers to an observation with numerous bubbles obscuring the heart sounds.
The experimental work carried out in the 1970s and 1980s has shown that bubbles can be detected in the circulation of healthy individuals after decompressions without any clinical signs of
DCS (
38). This confirms the early hypothesis by Behnke (1947), who postulated the existence of “silent bubbles” (
39). The paradigm of “bubbles =
DCS” appears not to be true in many, if not most cases, and recent research focuses on the pathophysiological cascade that can be started by the
in vivo gas phase (bubbles) in the different tissue compartments and the dose-response relationships leading to clinically evident
DCS manifestations. This explains why the demonstration of VGE in the cardiac chambers correlates poorly with the development of
DCS. A significant proportion of subjects do have detectable VGE, but do not develop
DCS and some develop
DCS without evident VGE (
38). As more research data becomes available, there is a trend to recognize certain degree of
DCS as a normal physiological response to a defined time-pressure profile environment with the
caveat of possible individual predisposition and other factors.
Terminology
The clinical syndrome of DCI was first recognized in the diving/compressed air environment and later found recognition in the area of aerospace medicine; this explains the wide variety of terms used to describe the disorder and certain specific clinical manifestations. The term
decompression sickness is a direct translation from the German term
Druckfallkrankheit, which was introduced by Benzinger and Hornberger in 1941 (
40). Currently,
altitude decompression sickness or simply
decompression sickness is the term most widely used and accepted in the aerospace medicine literature.
Older terms include aeroembolism, aeropathy, dysbarism, high-altitude diver’s disease, high-altitude caisson disease, mechanicobaropathy, aerobullosis, and aeroarthrosis.
Decompression illness is a term that was introduced to encompass
DCS and arterial gas embolism. There is also the distinct possibility for VGE to become arterialized either by crossing the pulmonary filter or crossing through a shunting mechanism from right to left side of the heart (
41). The term
DCI should not be used synonymously with
DCS to avoid confusion in an area with already broad terminology.
The typical clinical manifestations of
DCS have received idiomatic descriptions over time, the classical limb and joint pains are referred to as
bends, a term that was used by fellow workers to describe the particular gait—“doing the Grecian bend”—of workers emerging from caisson work during the construction of the piers of the Brooklyn Bridge in the 1870s (
26). Respiratory disturbances are commonly referred to as the
chokes, skin irritation as
creeps or
divers itch, and disturbances of the central nervous system (
CNS) with vestibular involvement have been labeled with the term
staggers.There are distinct and very important differences between hyperbaric (diving)
DCS and hypobaric (altitude)
DCS, despite many shared commonalities in history, pathophysiology, and nomenclature (
Table 3-4). This is a very important point as there is a tendency to indiscriminately transfer information and inferences from one field of research to another, which at times may be a valid thing to do, more often than not though may be unwise and not justified, thereby leading to potentially erroneous conclusions.
The operational significance of
DCS is different in hyperbaric versus hypobaric operations in that a diver will get
DCS after mission completion (ascent to the surface from depth), whereas an aviator will experience
DCS during his mission at altitude. Furthermore, the aviator will have the potential to endanger others if he or she loses control of his aircraft due to
DCS, whereas the diver will likely be putting only himself as an individual at risk.







 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access