Fig. 2.1
Summary of ageing changes in selected systems
2.1.1 Ageing Versus Disease
When discussing the notion of “normal ageing” or “physiological ageing”, one generally refers to the process of change that reflects alteration of organ structure and function with time alone and in the absence of supervening disease processes. This is referred to by some authors as primary ageing. Secondary ageing then refers to those aspects of the aged state that are attributable to disease. Disease-free individuals with the purely ageing-related changes suggested by the concept of primary ageing do not, and effectively cannot, in practice exist. Further, many changes in organs with ageing are arbitrarily defined as a disease when they progress to a point where they are recognizable clinically. Distinguishing between the effects of one of these degenerative diseases and “physiological ageing/primary ageing” becomes one of the preferred definitions in many cases.
2.1.2 The Impact of Ageing on Medical Care
Changes in the susceptibility of individuals to disease, as well as alterations in the way older people cope with metabolic disturbance, pathology and surgical or pharmacologic treatments have led to the discipline of geriatric medicine. Changes in physiology with age therefore will be built upon throughout the entirety of this text.
The distinct physiology of ageing can be summarized in the word “homeostenosis”. Coined by Walter Cannon, the influential American physiologist, this refers to a progressive loss of physiological reserve [1].
2.1.3 Why Do We Age?
Ageing represents cumulative changes in multiple organ systems. Rather than being a single process, the term is best thought of a reference to the net effects of accumulated degeneration in cells and tissues. A search for mechanisms that underlie ageing has been a focus of some interest in recent decades, and aspects of the biochemical and cellular processes which drive ageing have become somewhat better understood.
While there is no doubt all people experience ageing, there is a marked variability in the apparent pace of this process. This variability contributes to the wide range of lifespans observed across populations and suggests that at least some aspects of the physiological changes of ageing are influenced by an individual’s environmental exposures and peculiar genetic makeup.
2.1.3.1 Caloric Restriction and Other Interventions Known to Influence Ageing
Early insights into the potential for certain environmental factors to influence ageing derived from the effects of caloric restriction in rodents and other species in the laboratory [2]. In such studies, animals fed lower calorie diets compared to animals allowed to feed “ad libitum” exhibited longer total lifespans.
Calorie restriction is certainly associated with physiological adaptations including an altered metabolic rate. Sirtuin gene expression is influenced by caloric restriction and may in part be responsible for some aspects of altered physiological activity identified in calorie-restricted animals. While efforts have been made to replicate the effects of caloric restriction observed in the laboratory in humans, the long lifespan of our species and the difficulty of maintaining dietary interventions over long periods remain formidable obstacles to such clinical studies. At best surrogate markers of the effects of ageing are employed.
While the benefits and risks of caloric restriction in humans are unknown, there is compelling evidence for the adverse effects of malnutrition, which remains a major clinical concern worldwide and is prevalent even in developed countries among older people.
2.1.4 Cellular Processes and Ageing
2.1.4.1 Genetic Elements
Genes influence both lifespan and ageing. There is intriguing evidence that certain genes influence cellular senescence, a component of ageing, in many organisms and presumably in humans.
Rare genetic disorders described in humans suggest a prematurely aged state can be caused by mutations of specific genes, a group of disorders referred to as progeroid syndromes. Werner’s syndrome and Hutchinson-Gilford syndrome represent two examples of this unusual group of disorders. While these conditions are intriguing, whether they truly represent an acceleration of physiological ageing or merely resemble it remains unclear.
A number of mutations are associated with extended lifespans in laboratory models. Among the most studied of such genes are the previously mentioned sirtuins [3]. Originally identified in brewer’s yeast, these genes are now thought relevant to the process of ageing in many species. Sirtuins have been linked to ageing-related changes in the cardiovascular system [3]. Induction of sirtuin gene expression in response to environmental stressors seems to trigger metabolic and cell division changes in cells that are associated with longer usual lifespans in some species.
NF-kappa B influences gene expression both in inflammation and during ageing [4]. This gene may therefore be included among the “ageing” genes, and its expression presumably regulates some aspects of ageing physiology.
The complex relationship between an individual’s genetic code and the development of aged characteristics is clearly extremely complicated. To add to the complexity, it has recently been observed that epigenetic factors—that is, changes in the way DNA is regulated which are either somatotopically acquired or transgenerationally inherited and do not rely on DNA sequence—have been suggested to influence some aspects of the physiology of ageing [5].
2.1.4.2 Telomeres and Senescence
Cellular senescence refers to a response by dividing cells to stress. Activation of this state permanently prevents further cellular division and can be triggered by several different assaults on the cell, including telomere shortening and DNA damage [6].
The term telomere refers to sequences of DNA at the ends of chromosomes that progressively shorten with somatic cell division. Eventually this process prevents further divisions by inducing cellular senescence, creating a limit to the number of times somatic cells can divide—the Hayflick limit. This limit is presumed to be a component of ageing, though it is clearly only one factor of many [7] (Fig. 2.2).
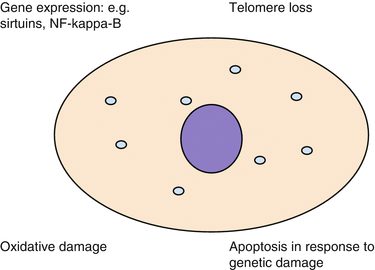

Fig. 2.2
Cellular processes influencing ageing
2.2 Ageing in Individual Systems
2.2.1 Cardiovascular System
Ageing represents the most important risk factor for diseases of the cardiovascular system. Changes in the cardiovascular system contribute to reductions in exercise tolerance and greater susceptibility to disease. Measurement of “normal function” across the lifespan, as in other aspects of ageing physiology, is hard to distinguish from the effects of clinical and subclinical disease.
Changes may be grouped in the following general categories: structural disease, disorders of function and the presence of disease more prevalent with ageing [8].
With ageing, there is an increase in the size of cardiac myocytes and, due to this, an increase in the relative cardiac wall thickness [9]. At the same time, the loss of numbers of cardiac myocytes results in reduced heart mass. Cardiac hypertrophy is therefore not an invariable result of physiological ageing [10]. As discussed earlier in this chapter, the effects of ageing on the heart and vascular system are influenced to some degree by the effect of sirtuin gene expression.
2.2.2 Respiratory System
Thoracic structural changes with ageing are associated with reductions in lung function. This includes changes in the ribs, spine and musculature. Wall compliance declines progressively in later life, presumably related to calcification of chondral rib insertion and changes in vertebral height. Kyphosis with ageing-related osteoporosis can cause reductions in FVC and FEV1 and an associated increase in AP diameter which effectively weakens the diaphragm.
Reduced gas exchange and increased stiffness of the lung both impact respiratory reserve, which declines progressively as an individual ages. Such changes may be readily demonstrated by use of serial spirometry. Changes in advanced age in the pulmonary parenchyma include alveolar dilatation which reflects changes in connective tissue composition [11].
There is a predictable reduction in lung elasticity with ageing which has consequences for lung function. The alteration seems to relate more to cross-links between collagen and elastin rather than the loss of such tissue from the lungs. The change in connective tissue arrangement produces dilation of the alveoli and the ducts producing a state that resembles emphysema and which is sometimes referred to by clinicians as “senile emphysema”. The production and function of surfactant do not seem to alter greatly with age [11].
Ageing-related changes in the properties of skeletal muscle cells, altering myosin production, patterns of fibre type and myocyte numbers all potentially contribute to reduced respiratory function which manifests as reductions in the strength of diaphragmatic contractions.
Disturbance of respiratory function during sleep is a particular problem and often is associated with pathological consequences.
2.2.3 Renal and Urological Systems
The urological system undergoes changes in structure and function with ageing that are expanded in the incontinence chapter of this text.
There is a reduction in the size of the kidney and number of glomeruli with ageing. As in other organs, the changes of physiological ageing are hard to distinguish from those of disease. Hypertension and even elevation of blood pressure in the normal range are associated with a greater rate of reduction in renal function [12]. Whether a fall in GFR is the normal physiological outcome of ageing or not remains a matter of debate. Renal blood flow in response to renal vasodilatation is reduced with healthy ageing [13]. Because loss of renal function is to some extent predictable by chronological age, this measure is usually incorporated into calculations estimating the glomerular filtration rate, such as the Cockroft-Gault and MDRD formulae.
Voiding difficulty is a common concern among older patients. Advancing age is associated with diminution of bladder capacity. There is an increase in the frequency of detrusor contractions. Despite this the effective expulsion urine falls with age so that the post-void residual increases. Urine flow rate decreases progressively with ageing. There is a detectable increase in neurotransmitter sensitivity in the bladder, accounting for the relatively high rate of adverse effects on bladder function observed with the use of medications acting on neurotransmitter pathways such as cholinergic drugs. There is a reduction in urethral pressure. Bladder ischemia may be an important factor in detrusor function change in some older people. There is fibrosis of the bladder wall which may be a consequence of ischemia. The bladder wall becomes thinner and the amount of muscle it contains diminishes.
The internal and external urethral sphincters are important for maintaining urinary continence. These structures are innervated by the sympathetic and parasympathetic nervous systems in the case of the internal sphincter and predominantly by spinal motor neurones in the external sphincter. Damage or deterioration of these control mechanisms in an older person is one factor resulting in greater risk of incontinence.
In men change in the size of the prostate with ageing frequently results in dysfunction of the lower urinary tract. Most men experience a benign increase in the size of the prostate with ageing which often results in symptoms of urinary retention and incontinence.
Antidiuretic hormone (ADH) is important in regulating fluid balance, and its secretion in the supine position changes with ageing in important ways with respect to the common problem of orthostatic hypotension.
2.2.4 Nervous System
No system is of greater importance to the diseases of ageing than the nervous system. Alterations in neurological function contribute to almost all the major physiologic alterations described in this chapter. Dysfunction of the nervous system is a component of all major geriatric syndromes (delirium, incontinence, falls and frailty). The effect of dysfunction of the brain in particular is a major reason for loss of independence and has a large and increasing effect on society as a whole.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree