Fig. 21.1
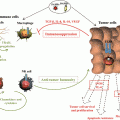
PDT induced effects. In tumors, cells loaded with PS upon excitation generate ROS species which leads to predominantly apoptotic and necrotic cell deaths. Tumor cell death is accompanied with complement cascade activation; proinflammatory cytokine activation; rapid neutrophils, DCs, and macrophages recruitment. Dying tumor cells and their debris are phagocytosed by phagocytic cells and DCs, which then migrate to the local lymph nodes and there differentiate into antigen-presenting cells. Tumor antigen presentation is then followed by clonal expansion of tumor-sensitized lymphocytes that home to tumor site and eliminate residual tumor cells
PDT has several advantages over other cancer treatment modalities currently in use. In addition to its selectivity and multiple application possibility, it is inexpensive with tolerable side effects. Moreover, it is rarely resistant to the observed treatments [26, 27]. More importantly, clinically approved PS does not accumulate in the cell nuclei and thus have limiting DNA damaging effects that can be by nature carcinogenic or can lead to the development of resistant clones. Several classes of inexpensive PS are commercially available and some are already approved to be used on patients. Most of the PS classes in use are of porphyrin or chlorin backbones or their modifications. With the newer PS classes, problems such as prolonged skin sensitization have been virtually eliminated [28]. Moreover, these compounds absorb in the region of visible spectrum, optimal for deep-tissue penetration. The list of benefits can be extended to include absence of the adverse effects of radiation and chemotherapies, no significant change in tissue temperature during illumination, preservation of the connective tissue at the PDT application site, thus minimal fibrosis induction, and improved cosmetic outcome. Clearly this is a very promising treatment modality that needs further translational and clinical studies.
In in vivo studies, the observed PDT effects can be attributed to several and interconnected biological and physiological effects. Depending on the PS concentration, location in the organism/tumor site, and applied irradiation dosage, PDT effects can be direct cell killing, occlusion of the tumor-associated vasculature, and modulation of the immune system, and sometimes cumulatively all of these effects can be observed. At the cellular level, both necrosis and apoptosis have been observed as the outcome of the PDT [17, 29–32]. It is a known fact that direct damage of the tumor cells and nearby vasculature initiates several cell-signaling cascades. In addition, damaged endothelial cells lead to formation of thromboses and consequently to vascular occlusion. In all these cases, the released cell fragments and cytokines trigger a range of inflammatory mediators which in turn activate the body’s defense mechanism, i.e., the immune response, which can be classified as innate or adaptive immunity. In essence, PDT treatment is generating a pronounced systemic effect as well as working in sync with the body’s natural defense mechanisms; the success of the PDT lies in the fact that it employs body’s “natural pathways” of defense.
PDT has been clinically applied to the treatment of early stage pulmonary, gastric, and esophageal carcinoma and has been examined for an application to other diseases such as retinal diseases [33, 34] or cardiovascular disorders [35, 36].
21.3 Closer Look Up at the PDT and Triggered Immune Response
In cancer treatment, one of the most important effects of PDT, besides tumor destruction, is that by the virtue of triggering an acute inflammatory reaction, it “activates” body’s immune system (Fig. 21.2). In fact, induction of a strong inflammatory reaction is the central paradigm of the antitumor effect of PDT. At the treatment locality due to PDT-induced oxidative stress, strong acute inflammation reaction and localized edema are generated [19, 37], i.e., PDT ends up producing a chemical (and subsequently a physiological) insult in the tumor tissue which is perceived by the body as a localized trauma. The next step is launching the protective mechanisms to reestablish tissue integrity and homeostasis at the treated/affected site [38]. At the onset, an acute inflammatory response is the principal effector. During this stage, the body is engaged in “containing the damage” – disruption of the homeostasis – which includes removal of damaged cells, and then promoting the healing process at the affected area, in order to restore normal tissue functions [38]. This elicited inflammation is nonspecific for the tumor antigen and is being orchestrated by the innate immune system [38]. The pattern recognition receptors are responsible for detecting the PDT-caused localized insult perceived as “altered self” [38]. PDT is responsible for speedy and prolific generation of “danger” signals, called damage-associated molecular patterns (DAMPs) or cell death-associated molecular patterns (CDAMPs), at the treatment site that get detected by the innate immunity [39–42]. At the onset of inflammation, the tumor vasculature undergoes significant changes and becomes adhesive for inflammatory cells (via over expressing selections) and permeable/leaky for blood proteins [38]. The inflammatory cells, first the neutrophils followed by mast cells, monocytes, and macrophages, infiltrate the PDT illumination site [43]. At this stage, the primary function of these cells is to “neutralize” the DAMPs/CAMPs by eliminating cellular debris, compromised tissue components, etc. [38]. The vascular occlusion, observed after PDT illumination, effectively “walls off” the damaged area, until it is removed by phagocytosis, thus preventing further spreading of the homeostasis disruption [38]. Studies have shown that depletion of these inflammatory cells or inhibiting their activity diminishes the therapeutic effect of the PDT [44–47]. Moreover, it is elucidated that interleukins IL-1β and IL-6 are amongst the most critical ones in this process [48, 49]. Also blocking the function of various adhesion molecules can render PDT ineffective [48, 49]. On the other hand, blocking the anti-inflammatory cytokines, IL-10 and TGF-β, can improve the PDT effect remarkably [50, 38].
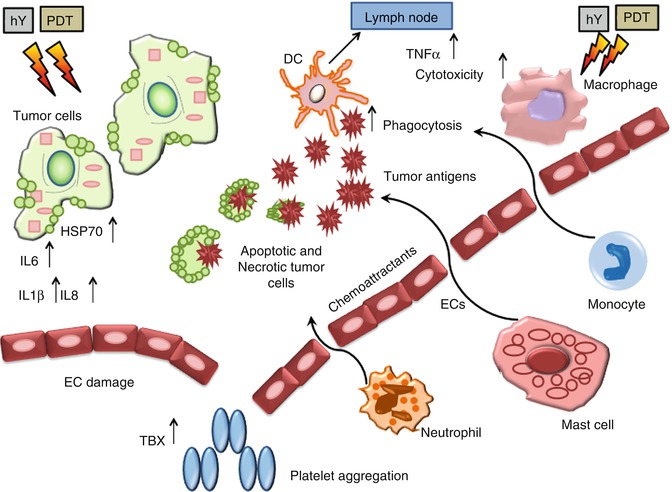

Fig. 21.2
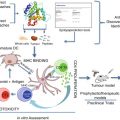
PDT induced inflammation. Damaging the endothelial cells (ECs) activates a cascade of events leading to local inflammation, vessel dilation, and platelet aggregation. Much of these effects are caused by the release of thromboxane (TBX), cytokines (such as interleukins IL1β, IL6, IL8, tumor necrosis factor-α), and infiltration of immune system cells (necrotic and apoptotic cells provide antigens to the DCs that migrate to lymph nodes)
21.4 Significance of PDT and Adaptive Immunity
Both preclinical and clinical studies have shown that PDT influences adaptive immune response in different ways; some regimens potentiate adaptive immunity, whereas others lead to immunosuppression. Although the precise mechanisms leading to the former or later response are not entirely clear, they appear to be PDT-regiment dependent [47, 51, 52]. Interestingly, PDT-induced immune suppression is mostly confined to cutaneous and transdermal treatments that involve larger surface areas [51, 53].
As previously mentioned, the efficacy of the PDT treatment strongly depends on the induction of antitumor immunity; research is showing that long-term tumor response is reduced or absent in immune-compromised mice [44, 54], whereas transfer of bone marrow or T-cells, from immunocompetent mice, results in improved PDT efficacy. In this process, recognition of the major histocompatibility complex class I (MHC-I) is critical for activation of CD8+ T-cells, thus tumors that lack MHC-I expression are resistant to cell-mediated antitumor immune reactions [55, 56]. Case in point, patients with vulval intraepithelial neoplasia (VIN) who lacked the MHC-I molecules did not respond to PDT treatment effectively as did patients expressing MHC-I [57, 58]; patients with positive PDT treatment response had increased CD8+ T-cell infiltration into the treatment site to differ with nonresponders, who lacked that effect.
The PDT effect over the immune system and more specifically induction of immune potentiation was demonstrated for the first time in the seminal study by Canti et al. [59]; the study proved that cells isolated from tumor-draining lymph nodes of PDT treated mice were able to pass on tumor resistance to naïve mice. Even more importantly, Korbelik et al. [60] in an in vivo study of murine tumors showed that PDT treatment generated an immune memory effect [60]. Multiple clinical studies support these lab research findings that PDT enhances the antitumor immunity effect. In clinical trials, PDT treatment of multifocal head and neck angiosarcoma showed reduction of untreated metastatic tumors owing to increased immune-cell infiltration into these untreated formations [61]. Further clinical phase I and II trials revealed promising results in proving the effectiveness of the PDT for induction of antitumor immunity effect [62–67].
21.5 Mechanism of PDT Immunologic Effects
Although the exact mechanistic pathways of immunologic activation are not entirely clear, there is a consensus that PDT activates both the humoral and the cell-mediated antitumor immunity systems. It is known by now that PDT efficacy is reduced and even null in the absence of CD8+ T-cell activation or their infiltration to tumorous sites [44, 68, 69]. Thus, it is imperative to have a clear understanding about the mechanisms of the potentiation of CD8+ T-cell activation due to PDT. One thing is clear, however, that PDT treatment induces acute local and/or systemic inflammation which culminates with antitumor immunity induction [52]. During this process, upon inflammation induction, dendritic cells (DCs) get matured and activated as critical components of tumor-specific CD8+ T-cell activation and, subsequently, antitumor immunity generation [70]. This chain reaction starts with DC activation (due to PDT treatment) followed by migration to the lymph nodes, where they activate the T-cells via presenting their antigens [49, 71]. At this stage, another class of T-cells may also be involved, the CD4+ T-cells, called also helper T-cells; they start dividing rapidly and secreting the cytokines that regulate and/or assist the immune response. The PDT-induced antitumor immune response may or may not depend on CD4+ T-cell presence [69, 72] and that role may be taken by the natural killer cells [69]; these are the cells bridging the adaptive immune system with the innate immune system, to differ from conventional T-cells (which recognize the peptide antigens presented by MHC), and these cells recognize the glycolipid antigens (however, once activated they can perform functions attributed to T-cells). In this cascade of cause-effects, it is believed that DC stimulation (thus increased ability to stimulate T-cells), at least partly, is due to dead and/or dying tumor cells [73]; it is known that PDT causes both cell death and cell stress [19, 74] and the initial activation of DCs at the PDT-treated locale is a result of DAMPs/CDAMPs recognition generated from the dying cells [75–77]. Recent studies have been looking extensively at the release patters of DAMPs after PDT [40, 41], and the most frequently expressed DAMP after PDT treatment seems to be the upregulation and translocation of the heat-shock proteins (HSPs) of the cell membrane [78].
21.6 Case Studies
For over a decade now, the Hamblin laboratory has been involved and has taken a leading role in elucidating mechanistic pathways of PDT-induced inflammation and antitumor immunity with the aim to trace novel immune mediated cancer treatment avenues stemming from PDT effects [79–89]. In the following section, we will discuss some of our findings, including the most recent study results, emphasizing the effects of PDT-generated inflammation and its reflection/implications in cancer therapy modalities.
It is widely accepted now that most deaths from cancer are caused by metastatic tumors; thus, our vision has been to develop methodologies that not only will destroy the primary tumor mass but also will activate the patient’s immune system to battle distant (untreated and may be not even detected yet) metastases [89]. It is well known now that removal of primary tumors via surgery and radiotherapy, which has immunosuppressive effect at high doses, renders micro-metastases to grow unchecked. On the other hand, after PDT treatment, there is an induction of an acute inflammatory response causing a massive regulated invasion of neutrophils [49], mast cells, and macrophages [90]. Not only that, but also, it has been shown that depletion of neutrophils in tumor-carrying mice decreased the PDT-mediated tumor treatment effect [54]. As discussed before, acute inflammation is implicated in attracting and activating DCs; as a result, they prime the tumor-specific cytotoxic T-cells (CTLs). In addition, it is well known that CTLs activity is not limited to the PDT treatment area alone and that they have a broader effective range [60]. Other groups have shown that low-dose cyclophosphamide (CY) can potentiate antitumor immunity in murine models. Suggested mechanistic explanations included depletion of suppressor T-cells [91], reduction of immunosuppressive cytokines [92], and anti-angiogenesis [93]; it has been generally accepted now that low-dose CY selectively depletes T-regs in mice, and by doing so, it increases both the priming and effector phases of the antitumor immune response [94]. In this crucial context, the authors reported, for the first time, that a combination of PDT with low-dose CY could cure a highly metastatic mouse tumor and could produce tumor-specific CTLs and potent memory immunity [89]. In this seminal work, we used J774, a highly metastatic reticulum cell sarcoma in BALB/c mouse, a highly aggressive, invasive, metastatic macrophage tumor, and PDT with benzoporphyrin derivative monoacid ring A (BPD). The CY was injected 48 h before light delivery. Our study demonstrated that PDT combined with low-dose CY generates a dramatic improvement in survival and numbers of cures. On the other hand, no cures but only some survival advantage were seen with each one of the components used separately, whereas when PDT was coupled with high-dose CY (as opposed to low dose), no additional benefit was observed. In comparison, with a combination treatment of BPD-PDT and low-dose CY, a long-term memory immunity generated allowed the cured mice to even reject rechallenging with tumorigenic doses of J774 cells. The observed long-term cures with only low-dose CY-PDT combination treatment suggests that in some tumor models, there is a kind of host factor which is counteracting the immune-stimulating effect of PDT. Judging by our flow cytometry results, this factor could be CD4+FoxP3+ T-regs, and the benefit of low dose CY could be due to their particular susceptibility to low-dose cytotoxic drugs. The effect of low-dose CY on the tumor was much more pronounced than the high-dose CY alone (Fig. 21.3). Our overall results are proving that the effects of CY on J774 tumor are due to the immunostimulatory effect rather than the traditional cytotoxic effect of the CY [89].
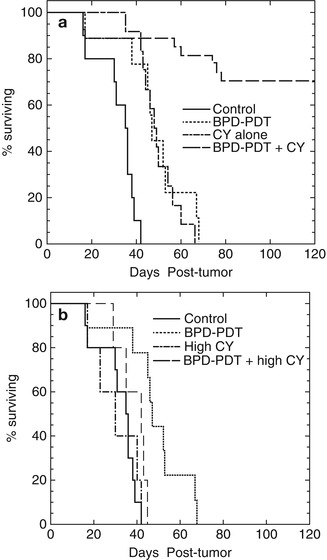

Fig. 21.3
Kaplan-Meier survival curves of mice undertreatment. (a) Plots represent no tumor treatment (as control), only PDT, low-dose CY, and low-dose CY+PDT. (b) Plots represent no tumor treatment (as control), only PDT, high-dose CY, and high-dose CY+PDT. Mice were killed in cases when the primary tumor diameter reached 1.5 cm or body weight dropped >15 % (Adapted with permission from Proceedings of National Academy of Science: Castano et al. [89])
It is widely accepted now that cancer treatment involving PDT modality is effectively engaging both arms (innate and adaptive) of the immune systems via stimulating the release or expression of various proinflammatory mediators [19, 37, 49, 75, 95, 96]. As a result, a powerful acute inflammatory response is launched causing accumulation of extensive numbers of neutrophils and other inflammatory cells at the PDT-treated site attacking the cancer cells [37, 43]. The fact is that this cycle is not only a powerful tool in eliciting direct antitumor effects [97–99], but as importantly, it is stimulating the cells to release secondary inflammatory mediators (including the cytokines IL-1β, TNF-α, IL-6, and IL-10 and prostaglandins, histamines, leukotrienes, etc. [100]). The one area needed to be further explored was to study the local treatment effects on eliciting systemic immunological response, in particular, establishing the link between PDT-mediated immunity and tumor antigens expression. Our lab was the first to recognize this effect. The authors designed a study in which a pair of equally lethal BALB/c colon adenocarcinomas were used: first, CT26 wild-type (CT26WT), i.e., antigen negative, and, second, CT26.CL25 transduced with lacZ gene, thus expressing the tumor antigen β-galactosidase (β-gal). The idea was to study if PDT treatment would elicit a systemic antigen-epitope-specific antitumor immune response in otherwise identical cancer cells [86]. In this study, both used cell lines were equally lethal, and the level of β-gal expression was low enough to allow the tumor to grow without triggering any clinically significant immune response (often seen in cancer patients), thus only PDT application could generate significant differences in the therapeutic outcome and the observed elicitation of immune response.
The outcome was that PDT induced a local response in all β-gal antigen-negative CT26WT tumors, with clear reduction in size, but only until day 18 (Fig. 21.4) after that the regrowth took hold. The net result was only that the growth was stalled for 8–10 days. In the case of CT26.CL25 tumors, however, the difference was dramatic (Fig. 21.4); tumor reduction was not only complete after day 20, but most importantly, 100 % of these β-gal antigen-positive tumors stayed in remission during the complete trial period of 90 days [86]. During the study, it was also observed that the PDT-induced immune response leads to elevated levels of released IFN-γ and TNF-α cytokines. Our study also shows that PDT can induce a very strong antigen-specific immune response, capable of generating memory immunity which allows mice to reject the rechallenge with the same antigen-positive cells. The induced immune response is potent enough to cause regression of a distant well-established antigen-positive tumor outside the treatment area [86] (Fig. 21.5). The presence of the activated antigen-specific effector CTLs was also confirmed. During the study, it was realized that regression of distant and untreated tumors took place in 70 % of the treated mice.
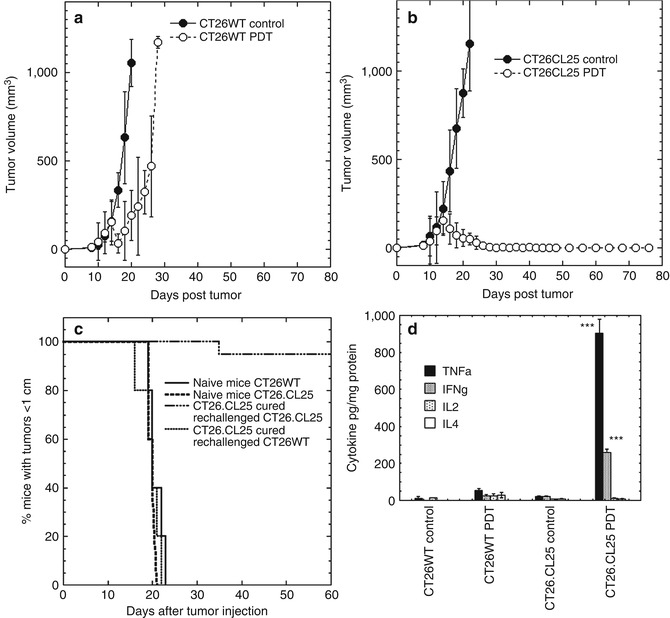
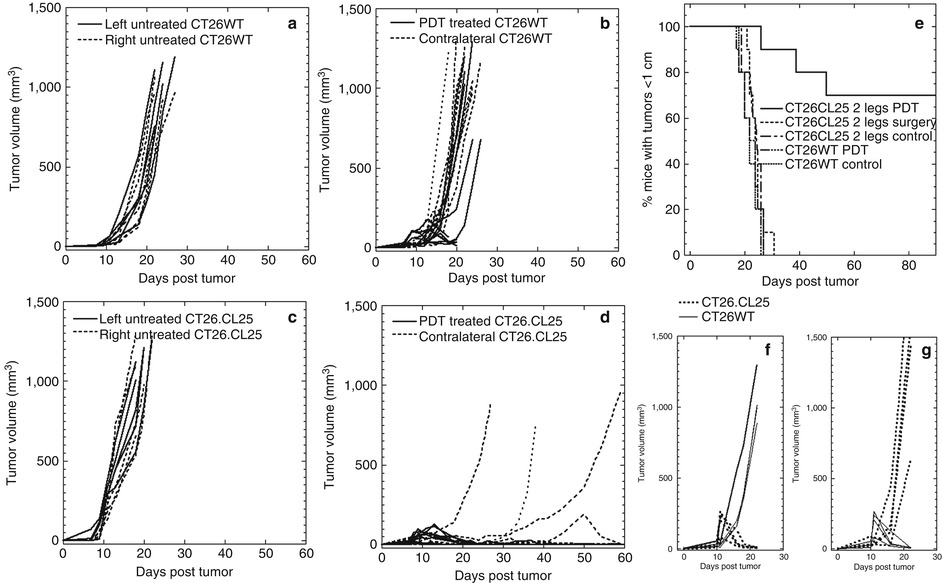

Fig. 21.4
In vivo PDT of tumor (1 leg model). (a) Mean tumor volumes of CT26WT tumors and (b) CT26.CL25 tumors; means of 10–15 tumors. (c) Kaplan-Meier survival curves of % of mice cured from CT26.CL25 tumors and rechallenged either with CT26.CL25 or CT26WT tumor cells. (d) Mean level of cytokines TNF-α, INF-γ, IL-2, and IL-4; measured 5 days after PDT in CT26.CL25 and CT26WT tumor-bearing mice and control mice (Used with permission from reference [86])

Fig. 21.5
In vivo PDT of tumor (2 leg model). Time courses of individual tumor volumes with two similar or mismatched bilateral tumors on the right and left legs. (a) Bilateral CT26WT tumors; right leg PDT treated. (b) Bilateral CT26WT tumors, untreated. (c) Bilateral CT26.CL25 tumors; right leg PDT treated. (d) Bilateral CT26.CL25 tumors, untreated. (e) Kaplan-Meier survival curves of % mice with tumor volumes smaller than 1 cm, in five groups: three groups with two similar bilateral CT26.CL25 tumors (one group untreated, one group with right leg tumor and PDT treated, and one group with right leg tumor surgically removed); two groups with two bilateral CT26WT tumors (one group untreated, one group with right leg tumor PDT treated). (f) Mismatched CT26.CL25 and CT26WT tumors; CT26WT treated with PDT. (g) Mismatched CT26.CL25 and CT26WT tumors; CT26.CL25 treated with PDT (Adapted with permission from Mroz et al. [86])
Moreover, our study demonstrated, for the first time, that tumor cells may escape PDT-induced immunosurveillance due to antigen loss. In clinical settings, it is known that some tumors escape from immune recognition and elimination; only now, we realized that this is happening due to tumor antigen loss. We also demonstrated that PDT-induced antitumor effects are abrogated when there is no functional adaptive immune response as in athymic nude mice (Fig. 21.6). Clearly, effective vascular PDT treatment can not only destroy a local tumor but also induce systemic strong antigen-specific antitumor immune response. And this immunity is so potent that it is capable to induce regression and destruction of distant, antigen-positive tumors outside the irradiation reach. The treatment also proved to be effective in inducing long-term immune memory effect, imprinting a resistance to rechallenge. Our study was successful in proving that the observed tumor-destructive effect was mediated by tumor antigen-specific cytotoxic T-cells, induced after PDT, which are capable of recognizing the immunodominant epitope of the β-gal antigen.
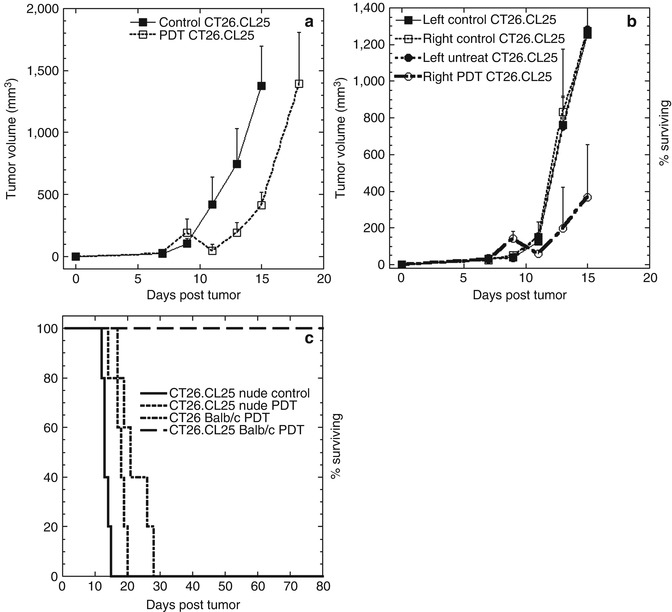

Fig. 21.6
(a) Tumor volumes of CT26.CL25 tumors PDT treated and untreated in BALB/c Nu/Nu immunocompromised mice. (b) Tumor volumes in bilateral CT26.CL25 tumors PDT treated and untreated in BALB/c Nu/Nu immunocompromised mice. (c) Kaplan-Meier survival curves of % surviving BALB/c and BALB/c Nu/Nu mice with either CT26.CL25 or CT26WT tumors, PDT treated. Non-treated BALB/c Nu/Nu mice with CT26.CL25 tumor is used as control (Adapted with permission from Mroz et al. [86])
To examine antigen-specific PDT-induced antitumor immune response in a more clinically relevant tumor model, the authors designed a separate study, where a naturally occurring cancer antigen, the P1A, a mouse homologue of human MAGE-type antigen, was employed [101]. We decided to use this specific cancer-testis antigen, since it is not only a well-established one, but more importantly, it is mostly expressed in testis and cancers and only at very low levels in other tissues [102–105]; P1A antigen-positive mouse mastocytoma P815 wild type (parental) and P1A antigen-negative P1.204 (P815 derived) cell lines were compared.
Murine methylcholanthrene-induced mastocytoma P815 cancer cells are known to generate very interesting immunologic response patterns. The significance of P815 antigen arises from the fact that it shares many characteristics identified in TAA genes in human, such as those belonging to melanoma MAGE family and other tumors [106]; these antigens are not expressed in most mature tissues with the exception of testis and placenta [107]. It is known that P815 can elicit CTL response against at least four distinct antigens: AB, C, D, and E [107–115]. It appears that the main CTL response against P815 tumor is geared towards AB and E antigens [111]. Also, it has been shown that T-cells isolated from DBA/2 mice inflicted with P815 tumor primarily recognize either antigen AB or C-D-E, but not both [116]. Moreover, the two epitopes of the P815AB, P815A, and P815B are recognized by two different CTLs. Another gene code for P815E and a different CTLs recognize its antigen. On the other hand, P815-derived P1.204 cell line is an immune system escape variant [117]; it has lost the P815AB antigen and only retains the P815E antigen.
During in vivo experiments performed by the authors, the majority of mice with P815 tumors revealed regression upon PDT irradiation and no recurrence during the trial period of 90 days. In stark contrast, mice with P1.204 tumor did not respond with tumor regression but rather with progression. The difference in response between the two tumor types was hypothesized to be due to differential triggering of immune response. To confirm the PDT-generated long-term immune system “activation” in this clinically relevant tumor model, we rechallenged the cured mice with the same tumor from which they were originally cured. Only mice cured for P1A antigen-positive P815 tumors rejected the rechallenging, while all the naïve mice injected with either tumor cell type grew tumors. The implication of the finding is that P1A antigen-positive P815 tumors, after PDT treatment, develop strong and robust enough immune response that prevents tumor growth upon challenging with a tumorigenic dose of cells.
In the ex vivo study, the extent of host antitumor immune response induction, as a result of PDT treatment of P1A antigen-presenting P815 mastocytoma cancer cells, and whether the antigen is activating T-cells before and/or after PDT, was looked into. The answer for that was provided by the cytokines secreted from CD4+ and CD8+ T-cells. Our results showed that PDT of P1A antigen-positive tumors led to marked increase in IL-2 and TNF-α levels. Moreover, we were able to identify a population of CD8+ T-cells that were able to recognize the LPYLGWLVF epitope of P1A antigen. In addition, in nude mice (lacking an adaptive immune system) bearing the P1A antigen-positive P815 tumors, antitumor effectiveness of PDT is curtailed to nil. Interestingly, their survival can be significantly prolonged by adoptive transfer of activated lymph node cells isolated from PDT-treated immunocompetent mice bearing the P815 tumor.
The initial escape of P815 tumors from immunosurveillance (and accordingly response) is documented to be due to antigenic loss [21, 39, 40]. It has been shown [110] that there are three different escape mechanisms employed by P1A tumors, presenting the peptide antigen LPYLGWLVF (expressed in different tumor models), for avoiding immune response: in P815 tumors, all progressions occur due to antigenic loss, while in J558 tumors, all progressions take place due to antigenic drift (antigen mutation [39]), whereas all progressing methA tumors develop resistance to CTLs.
Our study confirmed that if an antigen is expressed in a tumor tissue, PDT may be more successfully applied in patient population containing tumors positive for a particular antigen. Secondly, even though many solid tumors show heterogeneous expression of tumor antigens, it has been shown that de novo induction of tumor antigens in these tumors may represent a novel means to break tumor escape mechanisms [40]. Thirdly, combination of PDT with various tumor antigen expression enhancement and their presentation via MHC class-I, may have beneficial treatment effects for those cancers that are otherwise untreatable.
Application of PDT for localized microbial infections, especially those caused by multiple-drug-resistant bacteria, is a very promising alternative modality to antibiotics, particularly in intractable microbial infection situations; bone or joint infections caused by multidrug-resistant bacteria are extremely intractable. Moreover, treating orthopedic infectious disease (such as osteomyelitis, arthritis) can be problematic due to the aseptic nature of joints, bones, and cartilages. For such cases, we looked into the induction of protective innate immune response due to PDT treatment and observed that the process germinated through neutrophil accumulation.
It is well known that bacterial phagocytosis by innate immune cells, such as neutrophils, plays a critical role in the elimination of invading bacteria, especially Staphylococcus aureus [118–120]. Malfunction of the phagocytic immune system, therefore, renders the host susceptible to bacterial infections [121]. If a treatment impairs the function of phagocytes in combating microbial infection, the efficacy of the antimicrobial treatment might be reduced, resulting in deterioration and prolongation of the infection. We established a murine chronic MRSA arthritis model using a combination of bioluminescent MRSA and resin microparticles, which allowed sequential noninvasive optical evaluation of the course of infection in an individual mouse and enabled us to carry out a detailed examination of the PDT effects in an efficient manner [81]. We established that administration of anti-GR-1 (anti-neutrophil) antibody eliminated the therapeutic effect of PDT, indicating that the therapeutic PDT using methylene blue had a curing effect for bacterial infection via the attraction and accumulation of neutrophils into the infected region [81].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree