Photodynamic Ablation of Musculoskeletal Tumors
Jacob Bickels
Yair Gortzak
Yehuda Kollender
BACKGROUND
Malignant tumors of the musculoskeletal system, as many other solid tumors, require a true and wide surgical resection for their complete removal. The term wide margins currently used by orthopaedic and surgical oncologist refers to the margins of normal tissue surrounding a visible tumor mass, usually a few centimeters in thickness. However, such resection does not guarantee cure as microscopic disease is left throughout the surgical field beyond this wide cuff of tissue.
Adjuvant treatments for surgery are, therefore, required, and patients who undergo resection of malignant musculoskeletal tumors are usually referred for treatment with radiation therapy and/or chemotherapy upon their recovery from surgery. These adjuvant treatment modalities were shown to be effective in lowering the likelihood of local tumor recurrence. However, they are associated with a most considerable rate of complications, local and systemic, as they are not tissue specific and harm healthy tissues and organs as well.
INDICATIONS
Photodynamic ablation (PDA) allows specific tumor kill and therefore may provide a treatment option for the microscopic disease remaining in the surgical field following tumor removal. It is defined as the administration of a nontoxic drug or dye, known as a photosensitizer (PS), either systemically, locally, or topically to a patient bearing a tumor. The PS has specific affiliation to the tumoral tissue, and following its accumulation within the tumor cells, the tumor site is illuminated with a visible light, which excites the PS to generate cytotoxic species and consequently cause tumor cell death and destruction of tumor.
The realization that the combination of nontoxic dyes and visible light could kill cells was first made by Oscar Raab, a medical student working with Professor Herman Von Tappeiner in Munich in 1900. While investigating the effects of acridine dyes on the protozoa that causes malaria, he made an incidental discovery that the combination of acridine red and light resulted in protozoal death.12 He postulated that the effect was caused by the transfer of energy from light to the chemical, similar to the process of photosynthesis seen in plants after the absorption of light by chlorophyll. This discovery led to the first therapeutic medical application in which topical eosin, combined with white light illumination, were used to treat various skin tumors. It was soon realized that oxygen is required to allow this chain of reactions, and the term photodynamic action was used to describe this phenomenon.
ANATOMIC CONSIDERATIONS
Over the last few decades, PDA has been studied and used in a large variety of tumors. However, the use of PDA in the field of orthopaedic oncology was practiced only by a small group of enthusiastic surgeons.
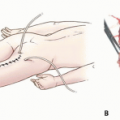
Matsubara et al10 reported on eight patients who had either bone or soft tissue sarcomas of the forearm and underwent intralesional resection of their tumor followed by PDA using topical administration of acridine orange (AO). Destruction of the microscopic disease that was left in the surgical field was done in three consecutive steps:
The surgical field is illuminated with blue light, which excites the AO within the residual tumoral tissue to emit green fluorescence. The tissue emitting this green fluorescence is detected with a designated surgical microscope and removed using an ultrasonic surgical scalpel.
Illumination of the surgical field with unfiltrated light from a xenon lamp
Single session of radiation therapy at a dose of 5 Gy, given immediately after surgery
This group of 8 patients was compared to another group of 10 patients who underwent wide tumor resection with adjuvant radiation therapy. The rates of local tumor recurrence were 12.5% and 20%, respectively, and patients who underwent marginal tumor resection with PDA had considerably better function.2
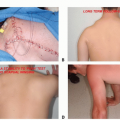
We are currently studying the use of PDA with 5-aminolevulinic acid (5-ALA) in the management of desmoid tumor and other benign and malignant soft tissue tumors, which have fibrous element in their structure. Desmoid tumors, also known as fibromatoses, are locally aggressive, soft tissue lesions. Extra-abdominal lesions appear between puberty and the sixth decade of life, with the peak incidence between 25 and 35 years of age. These lesions are located in a variety of anatomic sites, with the shoulder girdle, chest wall, back, and thighs being the most frequent locations in descending order.7 Although desmoid tumors do not metastasize, they are locally infiltrative and their propensity for local recurrence following wide resection is well documented. Although wide local resection still remains the treatment of choice for the majority of patients, the postoperative local recurrence rates were reported to be as high as 30% and 33% at the 5- and 10-year posttreatment follow-ups, respectively.1 Rock et al13 reported 194 patients with desmoid tumor who were treated at the Mayo Clinic, of whom 132 (68%) had a local tumor recurrence. Positive margins of the resection are considered a strong predictor for local tumor recurrence.1,6
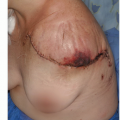
A locally recurrent desmoid tumor is a devastating clinical event. It usually requires extensive surgery for its removal and usually mandates adjunctive treatment (radiation therapy and/or chemotherapy). It is also associated with a
considerable loss of function and impaired quality of life. Because recurrence rates are so closely related to residual tumor tissue and positive margins of the resection, elimination of the microscopic disease left in the surgical field following wide tumor resection is key to lowering these high rates of tumor recurrence (FIG 1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree