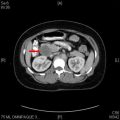
Pheochromocytomas (PCCs) and paragangliomas (PGLs) are rare but unique neuroendocrine tumors. The hypersecretion of catecholamines from the tumors can be associated with high morbidity and mortality, even when tumors are benign. Up to 40% of PCCs/PGLs are associated with germline mutations in susceptibility genes. About one-quarter are malignant, defined by the presence of distant metastases. Treatment options for unresectable metastatic disease, including chemotherapy, 131 I-MIBG, and radiation, can offer limited tumor and hormone control, although none are curative. This article reviews the inherited genetics, diagnosis, and treatment of PCCs and PGLs.
Key points
- •
Pheochromocytomas and paragangliomas (PCCs/PGLs), both benign and malignant, have high morbidity and mortality, especially when not properly diagnosed or treated.
- •
Blood pressure management, usually with the alpha-blocker phenoxybenzamine, is critical perioperatively for all patients and surrounding treatments for those with metastatic disease.
- •
Surgery is the only cure for PCC/PGL, but limited biochemical and tumor control of metastatic disease occurs with treatments, including 131 I-MIBG, chemotherapy, and radiation.
- •
Up to 40% of patients with PCC/PGL have germline mutations in susceptibility genes; therefore, all patients should be considered for clinical genetic testing.
- •
Future work is needed to identify predictors of metastatic potential and novel targets for therapy.
Introduction
Neuroendocrine tumors arising from the adrenal medulla and extra-adrenal ganglia are called pheochromocytomas and paragangliomas (PCCs/PGLs), respectively. PCCs/PGLs are rare tumors with an incidence of 2 to 8 per million. PCCs/PGLs occur in 0.2% to 0.6% of hypertensive patients and account for up to 5% of adrenal incidentalomas. Most PCCs/PGLs are benign yet associated with high morbidity and mortality secondary to hypersecretion of catecholamines and metanephrines leading to hypertension, cardiovascular disease and even death. Approximately a quarter of PCCs/PGLs are malignant, defined by the World Health Organization as the presence of distant metastases. Diagnosing PCCs/PGLs can be challenging, and treatments for metastatic disease are most often not curative. This review discusses the inherited genetics, diagnosis, and treatment of PCCs/PGLs.
Introduction
Neuroendocrine tumors arising from the adrenal medulla and extra-adrenal ganglia are called pheochromocytomas and paragangliomas (PCCs/PGLs), respectively. PCCs/PGLs are rare tumors with an incidence of 2 to 8 per million. PCCs/PGLs occur in 0.2% to 0.6% of hypertensive patients and account for up to 5% of adrenal incidentalomas. Most PCCs/PGLs are benign yet associated with high morbidity and mortality secondary to hypersecretion of catecholamines and metanephrines leading to hypertension, cardiovascular disease and even death. Approximately a quarter of PCCs/PGLs are malignant, defined by the World Health Organization as the presence of distant metastases. Diagnosing PCCs/PGLs can be challenging, and treatments for metastatic disease are most often not curative. This review discusses the inherited genetics, diagnosis, and treatment of PCCs/PGLs.
Genetics
Up to 40% of patients with PCCs/PGLs have a germline mutation in a known susceptibility gene, more than any other solid tumor type. Table 1 describes the susceptibility genes and the associated syndromes. The risk of PCC/PGL with each syndrome is discussed in the following sections.
| Gene | Protein | Function | Syndrome (Inheritance Pattern) | Metastatic PCC/PGL |
|---|---|---|---|---|
| Classic tumor syndromes | ||||
| NF1 | Neurofibromin | GTPase, which inactivates RAS to control the MAPK signaling pathway |
| 12% |
| RET | RET | Membrane tyrosine kinase receptor signals through PI3K |
| <5% |
| VHL | von Hippel Lindau protein | Ubiquitin ligase 3E activity which inactivates hypoxia inducible factors to control transcription of genes involved in angiogenesis |
| 5% |
| Succinate dehydrogenase complex genes | Complex II of the mitochondria respiratory chain that transfers electrons to the terminal acceptor ubiquinone and also converts succinate to fumarate in the TCA cycle | |||
| SDHA | Succinate dehydrogenase subunit A | Catalytic subunit |
| Low |
| SDHB | Succinate dehydrogenase subunit B | Catalytic subunit |
| 23% |
| SDHC | Succinate dehydrogenase subunit C | Anchoring subunit |
| Low |
| SDHD | Succinate dehydrogenase subunit D | Anchoring subunit |
| <5% |
| SDHAF2 (SDH5) | Succinate dehydrogenase cofactor AF2 | Cofactor for complex II leading to flavination of SDHA |
| Low |
| Additional susceptibility genes | ||||
| TMEM127 | Transmembrane protein 127 | Transmembrane protein in the endosome involved in the mTOR pathway |
| Low |
| MAX | MYC-associated protein X | Transcription factor heterodimerizes with MYC to control cellular proliferation, differentiation and apoptosis |
| Intermediate |
| EPAS1 | Hypoxia inducible factor 2A | Activates transcription of angiogenesis genes |
| NK |
| FH | Fumarate hydratase | TCA cycle enzyme which converts fumarate to malate |
| Possibly high |
| MDH2 | Malate dehydrogenase | TCA cycle enzyme which converts malate to oxaloacetate |
| NK |
Classic Tumor Syndromes
Neurofibromatosis type 1 (NF1) is an autosomal dominant syndrome found in 1 in 3000 individuals caused by mutations in the NF1 gene. The diagnosis of NF1 is made when patients meet at least 2 of the clinical criteria (see Table 1 ). PCCs, although not in the diagnostic criteria, occur at a higher frequency than in the general population. Approximately 5% of patients develop unilateral or bilateral PCC, and 12% of those are metastatic. The mean age at diagnosis of PCC is 42, similar to sporadic PCC. Guidelines suggest screening for PCC in patients with NF1 who have hypertension.
Multiple endocrine neoplasia type 2 (MEN2) is an autosomal dominant syndrome found in 1 in 30,000 individuals caused by activating mutations in the RET proto-oncogene. There are 3 subgroups of MEN2 described in Table 1 . MEN2A accounts for more than 90% of cases, whereas MEN2B and the rare familial medullary thyroid cancer subtype account for the rest. Fifty percent of patients with MEN2 develop PCC and half have bilateral disease. There are strong genotype phenotype correlations; therefore, screening recommendations for PCC vary depending on the mutation. Patients with MEN2A with RET codon 630 or 634 mutations and patients with MEN2B (most with RET codon 918 mutations) should begin screening for PCC at age 8. Patients with all other MEN2A mutations should begin screening for PCC at age 20. The mean age at diagnosis of PCC is between 30 and 40 years old, and the malignancy rate is less than 5%.
von Hippel Lindau disease (vHL) is an autosomal dominant syndrome affecting 1 in 36,000 births per year and caused by mutations in the VHL gene. vHL is defined by several benign and malignant tumors described in Table 1 . Unilateral or bilateral PCC occurs in 10% to 20% of patients, with rare reports of extra-adrenal or head and neck PGL (HNPGL). Similar to MEN2, there are strong genotype phenotype correlations. Patients with truncating mutations or exonic deletions in the VHL protein have a lower penetrance for PCC but a high penetrance for renal cell carcinoma. On the other hand, patients with missense mutations in VHL more frequently develop PCC when mutations are on the surface of the protein rather than the core. Screening for PCC in patients with vHL should begin at age 5 for families with high-risk mutations. The mean age at diagnosis of PCC is 30 years old, and approximately 5% develop metastatic disease.
Hereditary Paraganglioma Syndromes
Autosomal dominant mutations in succinate dehydrogenase (SDH), complex II of the mitochondrial respiratory chain, cause the hereditary paraganglioma syndromes. Mutations can occur in any of the subunit genes ( SDHA, SDHB, SDHC, SDHD ) or cofactor ( SDHAF2 also called SDH5 ) (see Table 1 ). SDHB is the most commonly mutated subunit leading to PCC/PGL. Mutation carriers develop extra-adrenal PGL but can also have PCC and HNPGL. The mean age at initial diagnosis of PCC/PGL is 32 years. SDHB mutation carriers with PCC/PGL have the highest risk of developing metastases at 23% based on a meta-analysis and systematic review. Long-term surveillance studies of mutation carrier families are needed to determine the true rate of malignancy and penetrance of disease.
SDHD is the next most commonly mutated subunit leading to PCC/PGL. Interestingly, only paternally inherited SDHD mutations cause disease phenotype, with extremely rare exception. SDHD mutation carriers develop multiple HNPGL but can have PCC and extra-adrenal PGL. The mean age at diagnosis of initial PCC/PGL is 33 years. The risk of malignancy is lower than 5%.
Mutations in the remaining SDH subunits occur much less frequently and also are associated with specific phenotypes. Eighty-one percent of the SDHC- associated tumors are HNPGL, 10% are thoracic PGL, and the remaining tumors are abdominal PGL and PCC. The mean age at first PCC/PGL is 38 years, and the risk of malignancy is very low. Only a few reported families with PCC/PGL have mutations in SDHA or SDHAF2 . Similar to SDHD mutations, SDHAF2 mutations have a parent-of-origin effect with paternal transmission of disease, and affected patients develop only HNPGL.
There are no guidelines on when to begin screening or what screening to do in asymptomatic SDHx mutation carriers. PCCs/PGLs have been reported in children as young as 5 years old. The full spectrum of clinical syndromes associated with SDHx mutations is still being defined but includes a risk of gastrointestinal stromal tumors, renal cell carcinoma, and pituitary adenomas (see Table 1 ). Therefore, most experts recommend annual biochemical screening for PCC/PGL starting between age 5 and 10 and full-body MRI screening for all associated tumor types every 2 to 5 years until more information is known.
Additional Susceptibility Genes
Other susceptibility genes have been implicated in PCC/PGL at low frequency (less than 2% of cases) including MAX ( Myc-associated protein X ) and TMEM127 (Transmembrane protein 127) , both of which are usually associated with PCC (see Table 1 ). Rare patients with PCC/PGL, with or without polycythemia and somatostatinomas, have somatic mosaicism for EPAS1 mutations, encoding a mutant hypoxia-inducible factor 2α. There are case reports of other susceptibility genes in the tricarboxylic acid cycle (TCA) cycle. A few families with germline fumarate hydratase , FH , mutations and one family with germline malate dehydrogenase, MDH2 , mutations have been found to have PCC/PGL. Clearly, dysregulation of the TCA cycle (by SDHx, FH, MDH2 mutations) predisposes to PCC/PGL, and it will not be surprising in the future to find rare families with PCC/PGL who have mutations in other TCA cycle enzymes.
In sporadic PCC/PGL, there are low rates of somatic mutations in the classic susceptibility genes, such as NF1, VHL and RET , but surprisingly, not in SDHx genes. Up to 10% of sporadic tumors have somatic mutations in HRAS . Recently, approximately 13% of PCCs/PGLs were found to have somatic mutations in ATRX , a chromatin-remodeling gene also mutated in pancreatic neuroendocrine tumors, and these mutations were associated with clinically aggressive PCC/PGL.
Given the high rate of inherited mutations associated with PCC/PGL, the Endocrine Society guidelines recommend referring all patients with PCC/PGL for consideration of clinical genetic testing. The clinical phenotype, as discussed previously, can guide the order of gene testing, as can the biochemical profile ( Table 2 ). As panel gene testing is becoming more cost effective, it is replacing single-gene testing in most centers.
| Biochemical Profile | Possible Gene Mutation |
|---|---|
| Noradrenergic | SDHx or VHL or sporadic (no mutation identified) |
| Adrenergic | RET or NF1 |
| Dopaminergic | SDHx (often SDHB ) |
| Methoxytyramine a | SDHx (or seen in metastatic disease even without a known mutation) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree