Fig. 13.1
A pharmacologic audit trail for clinical trial design. Solid boxes contain the conceptualized trial framework; dashed boxes demonstrate the example of a new therapy targeting androgen receptor (AR) splice variants (AR-sv) in castration-resistant prostate cancer (CRPC). CTC: circulating tumor cell; PSA: prostate-specific antigen
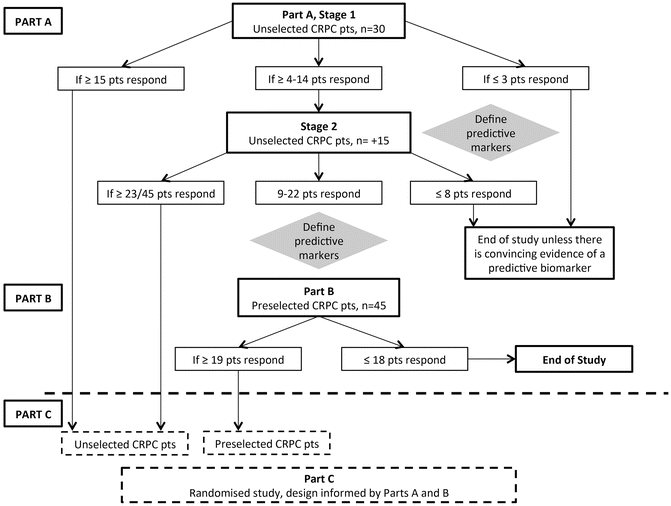
Fig. 13.2
The Phase II TO-PARP trial framework. CRPC: castration-resistant prostate cancer; pts: patients; n: number
Response Endpoints
Limitations in assessing response in CRPC have long been recognized. Neither PSA nor derived measures such as PSA change and PSA velocity have met surrogacy criteria for survival benefit [50]. Despite this, PSA remains a common measure of treatment response [41]. PSA is widely used in Phase I/II trials, although the mechanism of action of the therapy may render PSA an unhelpful marker of activity. For example, immunotherapies may demonstrate improved survival without impacting PSA kinetics [52, 53].
In the 1970s bone scintigraphy was adopted for the detection of bone metastases [54]. False negative scans due to lack of osteoblastic response or small lesion size, lack of specificity, and difficulties with reproducibility were quickly recognized [55]. Despite these issues, bone scans became part of the standard staging for men with prostate cancer. The PCWG2 recommended assessing progression based on the appearance of new lesions, rather than more subjective measures relating to tracer intensity, or individual lesion size or area [41]. More recent attempts to improve bone scan assessment have included computer-aided interpretation, which showed a good ability to reproduce the bone lesion area, intensity, and count assessments of a highly experience nuclear medicine physician [56].
The Response Evaluation Criteria in Solid Tumours (RECIST) were published in 2000 and aimed to standardize response assessment for soft tissue disease manifestations [57]. These criteria were incorporated in a modified form in the PCWG2 recommendations, including 3-monthly restaging with CT and bone scans. Alternative imaging modalities have been proposed and although the optimal modality and technique remains unclear, there is increasing enthusiasm for replacing the traditional methods of prostate cancer assessment. Whole-body DW-MRI has been shown to outperform Tc 99 m bone scanning and may be suitable to assess treatment responses [58]. 18 F-Choline positron emission tomography-CT (PET-CT) may also offer improved sensitivity and specificity in detection of prostate cancer metastases [59]. However more data are required to bring these imaging modalities into routine clinical practice and trial design.
Novel biomarkers for efficacy monitoring include CTC counts and biology-driven markers of treatment effect [50]. These strategies require a rigorous process to prove surrogacy, but can be useful to identify patients likely to benefit from targeted therapy. Encouraging results were observed in the COU-AA-301 trial, where CTCs in combination with LDH demonstrated survival surrogacy on an individual patient level [60, 61].
Conclusion
The past decade has witnessed strong interest in prostate cancer research, resulting in six survival-prolonging treatments for men with CRPC and a deeper understanding of the complex molecular drivers of progression. Molecular pathways of growth are being targeted using novel agents and combinations. Successful drug development requires a comprehensive strategy, from intelligent early phase trial design, through to appropriately designed and powered Phase III trials. Multiple valid designs and endpoints can be utilized, but selecting the best fit for the investigational target will maximize the likelihood of successful drug registration. Incorporation of translational science during early phase development can benefit both in the later stage trial design and in increasing our understanding of the underlying biology of CRPC.
References
1.
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC. Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst. 1997;89(15):1138–47.PubMed
2.
Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101(10):708–20.PubMedCentralPubMed
3.
Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17(10):1103–20.PubMed
4.
Eisenberger MA, Sinibaldi VJ, Reyno LM, Sridhara R, Jodrell DI, Zuhowski EG, et al. Phase I and clinical evaluation of a pharmacologically guided regimen of suramin in patients with hormone-refractory prostate cancer. J Clin Oncol. 1995;13(9):2174–86.PubMed
5.
Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010;375(9724):1437–46.PubMedCentralPubMed
6.
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–97.PubMed
7.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364(21):1995–2005.PubMedCentralPubMed
8.
Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–48.PubMedCentralPubMed
9.
Attard G, Swennenhuis JF, Olmos D, Reid AH, Vickers E, A’Hern R, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 2009;69(7):2912–8.PubMed
10.
Patel JC, Maughan BL, Agarwal AM, Batten JA, Zhang TY, Agarwal N. Emerging molecularly targeted therapies in castration refractory prostate cancer. Prostate Cancer. 2013;2013:981684.PubMedCentralPubMed
11.
Arbuck SG. Workshop on phase I study design. Ninth NCI/EORTC new drug development symposium, Amsterdam, March 12, 1996. Ann Oncol. 1996;7(6): 567–73.PubMed
12.
Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7): 1813–21.PubMedCentralPubMed
13.
Yap TA, Omlin A, de Bono JS. Development of therapeutic combinations targeting major cancer signaling pathways. J Clin Oncol. 2013;31(12):1592–605.PubMed
14.
Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28): 4563–71.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree