Atorvastatin
40 mg
Daily
Escitalopram
20 mg
Mane
Frusemide
40 mg
BD
Metoprolol
50 mg
BD
Paracetamol
1 G
TDS
Temazepam
10 mg
Nocte
Warfarin
1 mg
Nocte
OxyContin
5 mg (short acting)
TDS
Allopurinol
50 mg
Every second day
Isosorbide mononitrate
60 mg
Mane
Esomeprazole
20 mg
Mane
Nitrolingual spray
1 puff
PRN
Ramipril
1.25 mg
Nocte
Cetirizine
10 mg
Nocte
Digoxin
62.5 mcg
Mane
Metoclopramide
10 mg
½ h before meals
The family voices concern about her mortality risk and requests assessment for nursing home. The patient however requests to stay at home for ‘as long as is possible’. The examination was notable only for slight deafness; a large heart clinically, postural systolic blood pressure drop of 50 mmHg (causing symptoms); and a mild sensory peripheral neuropathy.
This case is a typical presentation seen by general, internal medicine and geriatrics practitioners. Although the history of the presenting complaint and detailed past history are not given, the medical list and its relationship to the presenting complaint raise a large number of concerns. Specifically this case highlights a number of issues around prescribing in the elderly including polypharmacy, possible compliance issues and communication issues between patient, specialists and general practitioners (GPs). There are specific pharmacokinetic (PK) and pharmacodynamic (PD) parameters that change in the elderly generally and which are likely to cause symptoms and impaired quality of life in this patient specifically. These changed PK and PD processes are not all or nothing processes, but a continuum across age, gender and comorbidity. Thus this chapter will focus on general principles for prescribers to consider when prescribing in the elderly.
It should be noted that most drugs used in clinical practice have never had their PK of PD studied in the elderly, specifically not in the over 75-year age group or in those with additional comorbidity. It is not a requirement of regulatory authorities to do so; thus most studies tend to enrol patients under 70 and without the comorbidity commonly coexisting in the elderly and which causes altered PK and PD.
Although not providing information on existing agents in clinical use and although not mandating actual geriatric data, the ICH/European Guideline Clinical Investigation of Medicinal Products in Geriatrics1 gives some guidance to industry around the registration of new active substances that are likely to have significant use in the elderly, either because the disease intended to be treated is characteristically a disease of aging or the population to be treated is known to include substantial numbers of geriatric patients or if there are reasons to expect that conditions common in the elderly (e.g. organ impairment, concomitant illnesses or medications) may alter the geriatric patient’s response (with regard to either safety/tolerability or efficacy) compared with that of the non-geriatric patient. In this 1994 document, it is stated that geriatric patients should be included in the Phase III database (and in Phase II, ‘at the sponsor’s option’) in meaningful numbers. However many ‘new’ therapies, for example, cancer therapies, may come to registration on Phase II data only. In practice, the geriatric data may be ‘simulated’ from other populations or translated from healthy geriatric volunteers rather than actual data from a geriatric population with common comorbidity. Further the PK data, if available, is usually based on single or short-term dosing, in distinction to the long-term use and with concomitant therapies, usual in the elderly.
In addition to the often limited trial data of these drugs in the elderly, it can be difficult for investigator-initiated studies, such as post-marketing pharmacovigilance studies in the elderly, to receive nonindustry funding to provide the much needed evidence. This may be because of the complexity of both the pharmacology issues and the heterogeneous population which can span a 40-year time period (ages 60–100 years). These studies would require large numbers of otherwise homogenous older people to reduce confounding. It can even be difficult to conduct large industry-sponsored randomized controlled trials in elderly patients as side effects and withdrawals are likely to be much larger than a younger healthier population, potentially threatening registration. Further many elderly patients have several different diseases and take many different medications that cannot be discontinued so that a patient can participate in a drug study. Therefore listing is more likely (and the clinical trial size much smaller) for a sponsor if the clinical studies focus on a homogenous, healthier and younger population likely to better tolerate the drug.
4.2 Pharmacokinetics
Pharmacokinetics describes how a person processes a specific drug after its administration. Route of administration route is very important as although there is often knowledge about the population relationship between plasma concentrations after, e.g. IV or oral dosing, the ratio may differ in a particular elderly patient depending on other comorbidity including gut function, type of diet, concurrent medication that affects gut transit time (such as prokinetic agents) and chemical issues such as concurrent PPI administration. Timing and frequency can also affect target drug concentrations.
In addition, every therapeutic has a pharmacokinetic profile based on specific parameters such as age, sex, weight, body mass index, hepatic function and renal function, inter alia. The effect of sex may actually be more related to body composition than sex; however sex can be the important covariate to understand if body composition parameters are not available.
Overall the pharmacokinetics of most medications in elderly adults has not been studied in enough detail to recommend use or at least correct dose in the elderly or very elderly. Therefore having enough knowledge of the principles of pharmacokinetics (absorption, distribution, metabolism and elimination) in general and in the particular patient in front of you can help make reasonable predictions about likely pharmacokinetics to support a decision about dosing and timing.
4.2.1 Absorption of Oral Medications
Changes in the elderly gut that affect the actual or the time to peak concentration. This can affect efficacy (e.g. in drugs that need rapid achievement of high Cmax).
These changes are:
- 1.
Reduced GI motility increasing absorption
- 2.
Reduced GI blood flow reducing absorption
- 3.
Increased extent of absorption of drugs that undergo first pass metabolism, as seen with nitrates and the lipophilic beta blockers (e.g. metoprolol)
- 4.
Reduced gastric acid secretion causing more alkaline gastric pH reducing drug absorption
- 5.
Swallowing difficulties leading to erratic drug absorption
- 6.
Poor and inconsistent nutrition leading to altered absorption of both lipid and water-soluble drugs
- 7.
The use of (per enteral or nasogastric) feeding tubes affecting rate and extent of absorption
- 8.
Medication use may contribute greatly to these changes e.g. concurrent use of antacids and overuse of proton pump inhibitors
The net effect of these changes is difficult to predict and may vary depending on the nature of the drug being prescribed.
4.2.2 Distribution
This is the site of drug distribution after the drug is either injected (for intravenous) or absorbed and passed through the liver (for oral medication). It is estimated using a parameter named the apparent volume of distribution of a drug. This is calculated as the amount of drug in the body divided by the concentration of drug measured in a biological fluid.
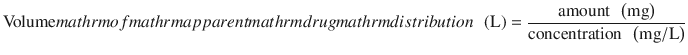
Some drugs are widely distributed into tissues, body fluids using diffusion, active transport using pumps such as the P-glycoprotein pumps and other energy-dependent pumps as seen in the gut, kidney and brain, for example. Drugs that need to distribute into the brain for activity such as opiates must cross the blood–brain barrier.
The volume of distribution (Vd) of a drug is affected by the lipid or water solubility of a drug and the amount and proportion of water and fat present in that particular elderly subject. Standard population volume calculations are provided by the drug sponsor during the regulatory process. However these are usually derived in healthy and young people. Most elderly patients have a Vd different to younger people due to different body composition. Within the elderly group itself, Vd is also changeable, for example, in people with liver or renal impairment or heart failure. Estimating the volume using basic pharmacology principles however enables a more appropriate dose to be chosen, assuming the desired concentration at the site of action is known.
Even in the ‘healthy’ elderly, changes occur in the body composition of water and fat. Depending on the physiochemical factors of the drug (e.g. whether it is lipophilic or not), this can affect how a drug is distributed. In both men and women, as the body ages, muscle mass declines and the proportion of body fat increases. Thus drugs that are fat soluble may be relatively more widely distributed compared with a young person. For drugs distributed in the blood, the volume of distribution may be reduced. This effect is observed with many fat-soluble benzodiazepines, requiring dosing reductions in the elderly.
The aging process also is associated with a theoretical reduction in total body water, which can affect the volume of distribution of water-soluble drugs. Older adults in general produce less albumin, which binds drugs in the blood. Reduction in protein binding (e.g. from low albumin) can result in an increase in free drug concentration. As the free drug concentration increases (compared with bound drug), more drug becomes available to bind to receptors or cross membranes, thus increasing the pharmacologic effect in an elderly individual. However, in those situations, an increase in free fraction results in increased excretion, so over a dosing interval, the free fraction should return to its normal concentration; the lower total amount (which is often what is measured in automatic laboratory assays) reflects this. This is supported by well-conducted clinical pharmacology on the clearance of free phenytoin in the elderly—showing that although there is a trend towards reduced clearance of free phenytoin in the elderly, it was not significant [1]. This also shows the importance of measuring free concentrations of drugs that are highly protein bound.
All of these effects can influence how a drug is distributed and the resultant plasma concentrations achieved. Without a resultant change in dose to account for this, volume of distribution thus determines whether a pharmacologic or adverse effect can occur. As an example, if the volume of a drug is reduced, then the loading dose that is necessary to achieve a desired concentration is reduced and the half-life of the drug (the time it takes for the blood concentration to decline by 50%) may be altered. Failure to take these changes into consideration can result in drug toxicity, as is sometimes seen when a standard loading dose of digoxin is used. Changes in the half-life of a particular drug also will determine the specific dosing regimen for a patient. If the Vd of a hydrophilic drug (e.g. heparin) is increased, e.g. in heart failure, then the opposite effects occur.
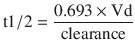
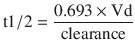
Consideration of how a drug’s volume of distribution may be altered in an elderly patient is an important component to help determine the proper drug dose for an individual. Drugs that have undergone study in elderly patients to determine how the volume of distribution will change because of aging, of which there are very few, can be dosed more precisely in this population. For drugs lacking such information, the dose should be started low and increased slowly to a clinically relevant target or to a well validated surrogate of a specific effect.
i.e. START LOW GO SLOW
4.2.3 Metabolism
The majority of drug metabolism occurs in the liver. A small amount occurs in the gut wall, the renal cortex and other organs such as heart and lung. Thus changes to these organs directly and indirectly via changes in blood flow, particularly the liver will have significant effects on drug metabolism.
Effects on the liver are multifactorial as the liver synthesizes proteins to bind to drugs, synthesizes enzymes to reduce or oxidize drugs to metabolize them and adds variety of water-soluble chemicals to lipid-soluble drugs to enable renal clearance. The effect on drug liver clearance is determined by the blood flow through the liver (Q) reflecting drug delivery to the liver, the fraction of drug in the blood that is free or not bound to plasma proteins and capable of interacting with hepatic enzymes (f) and the intrinsic ability of hepatic enzymes to metabolize the drug, which is commonly referred to as ‘intrinsic clearance’ (Clint). Intrinsic clearance is the ability of the liver to remove drug in the absence of flow limitations and binding to cells or proteins in the blood, both of which can be affected with synthetic impairment and liver cell integrity.

![$$ \mathrm{Hepatic}\kern0.50em \mathrm{clearance}:\mathrm{Cl}\left(\mathrm{h}\right)=Q\left[\left(f\times {\mathrm{Cl}}_{\mathrm{int}}\right)/\left(Q+f\times {\mathrm{Cl}}_{\mathrm{int}}\right)\right] $$](http://oncohemakey.com/wp-content/uploads/2020/03/A371068_1_En_4_Chapter_Equc.gif)



