Introduction
As the global population ages, the ongoing impact of osteosarcopenia is poised to strain or overwhelm healthcare systems around the world . To combat this, effective treatment strategies are required beyond those that are already in place. The management of osteosarcopenia is an ongoing challenge in clinical practice. While the pharmacological strategies to manage osteoporosis are well defined, there are no approved compounds to treat sarcopenia, nor muscle and bone together. This necessitates an approach in which the clinician treats each component of the disease individually, i.e., osteoporosis medications, alongside adjunct therapies and nutritional approaches to improve muscle function and fall-related outcomes . While this is not ineffective, there is growing interest in the identification of drug compounds that target both muscle and bone individually. While in its early days, there are a small number of agents in preclinical and clinical trials that may be able to improve function and outcomes across both muscle and bone simultaneously ( Table 1 ). This chapter will discuss the current state of the evidence on compounds affecting both muscle and bone, as well as the challenges they face.
| Pharmacological agent | Osteoporosis | Sarcopenia |
|---|---|---|
| Denosumab | Significant improvement in BMD at lumbar spine, hip, and radius. Reduces fracture risk. | Reduction in falls in the Denosumab treatment group of the FREEDOM study. No evidence of an effect on muscle function in that study. Improves muscle strength and insulin sensitivity and reduces falls and fear of falling in high-risk older persons. |
| Testosterone | Intramuscular testosterone increased lumbar spine bone density in men. | Testosterone in older men with decreased testosterone levels and muscle weakness can improve muscle mass, strength, and physical performance. |
| SARMs-Ostarine | Increases lean body mass and muscle power in elderly men and postmenopausal women. | Improves bone mass and architecture in ovariectomized rats. |
| Growth hormone | Metaanalysis of 7 RCTs and one extension trial concluded that growth hormone may not improve bone density but decrease fracture risk in women with age-related bone loss. | Low-growth hormone levels with age contribute to a decrease in lean body mass and increase adipose tissue. |
| Antimyostatin antibodies | Antimyostatin antibody in combination with resistance exercise improved bone health in rats. |
|
Targeting the endocrine system for muscle and bone anabolism
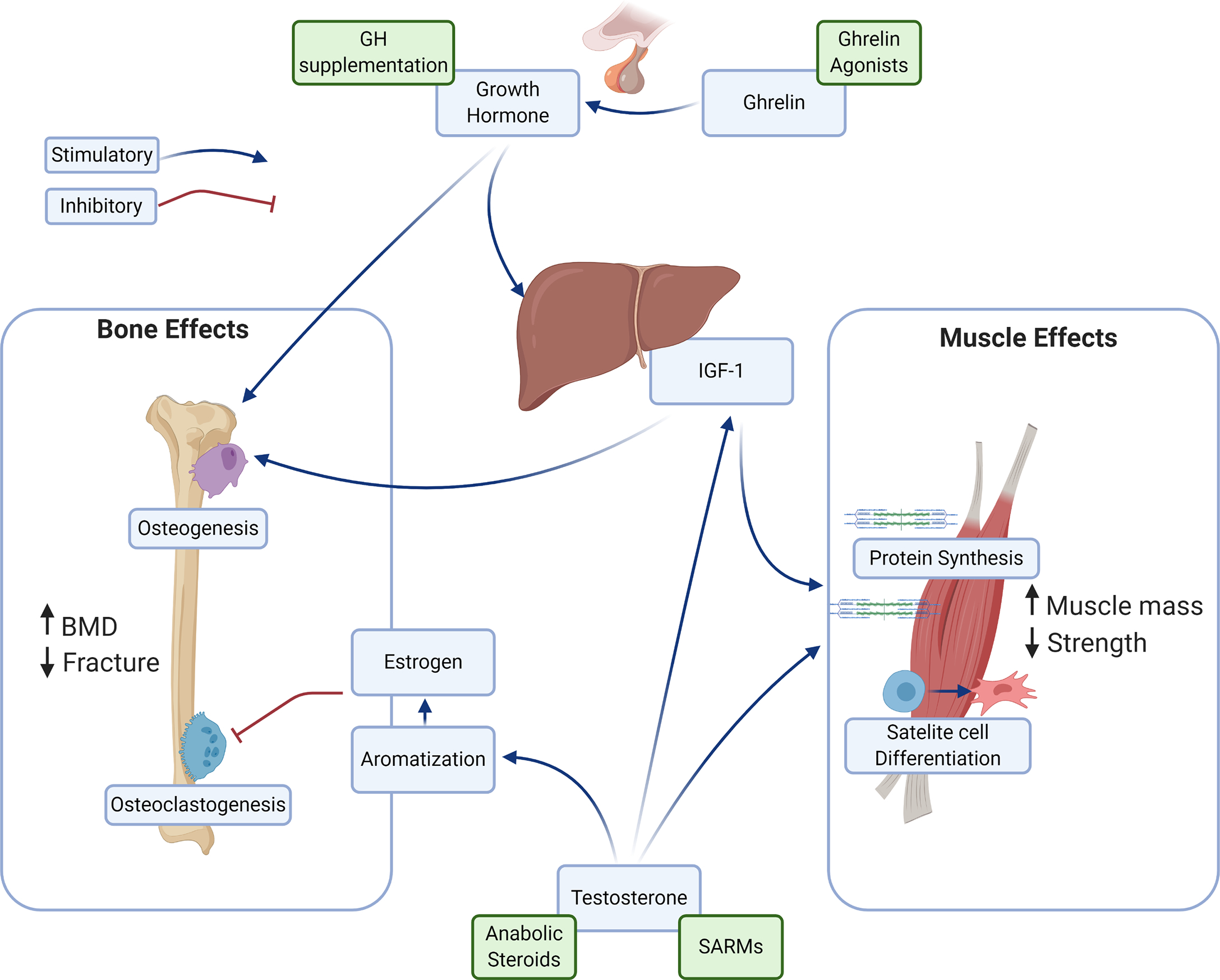
The central role the endocrine system plays in the development and maintenance of musculoskeletal tissues has driven interest in the exploitation of these factors for the treatment of muscle and bone disease. Hormones such as testosterone and growth hormone, as well as synthetic compounds that manipulate their physiologies have all been investigated as agents amenable to drug development. The pathways evaluated in research on pharmaceutical manipulation of osteosarcopenia are summarized in Fig. 1 .
Androgens
The gonadal hormones play a major role in the development of all musculoskeletal tissues and have been the focus of research into potential compounds that could be used to improve bone and muscle mass in older adults. Estrogen and other compounds that selectively interact with its receptor in different tissues, called selective estrogen receptor modulators (SERMs) were commonly used historically in the treatment of postmenopausal osteoporosis. However, from the perspective of osteosarcopenia, they are only a solution to half of the equation, with minimal effects on muscle . As is well understood from a developmental perspective, androgens have a much stronger impact on the development of both bone and muscle mass, and this is reflected in their therapeutic use in osteoporosis and sarcopenia. Men with hypogonadism develop both osteoporosis and muscle wasting, and treatment with testosterone reverses these effects lending support to its use as an anabolic treatment for musculoskeletal disease .
The mechanisms by which testosterone imparts its anabolic effect vary based on the tissue. In bone, it undergoes aromatization and is converted to estrogen, where it inhibits osteoclast formation, while in muscle it acts directly to increase the synthesis of protein, leading to an overall increase in both muscle size and function. It also acts to increase the secretion of insulin-like growth factor-1 (IGF-1), which synergistically enhances the anabolic responses . These actions are consistent in their magnitude throughout the lifespan, further supporting its use as a therapeutic strategy.
Testosterone has been shown to be effective in increasing both bone and muscle mass, particularly in men with hypogonadism. A systematic review of 8 trials, enrolling 365 men with the hypogonadal disease found an 8% increase in lumbar spine bone mineral density with intramuscular testosterone treatment . However, it found no association with femoral neck BMD, and none of the studies examined fracture risk, leaving large questions unanswered . These findings have been supported by other reviews, which also found no evidence to suggest that testosterone therapy could decrease the risk of fracture . A more recent study of 211 men who received testosterone treatment for a year not only had similar findings showing increased spinal BMD but also demonstrating an improvement in femoral bone mass . Another study of 100 obese men on very low-calorie diets who received intramuscular testosterone therapy for a year, found that it reduced serum c-telopeptide (CTX), a marker of bone resorption, suggesting it may aid in maintaining bone mineral density. However, they also found that while the testosterone treatment initially increased the bone formation marker N-terminal propeptide of type 1 procollagen (P1NP), it was marginally lower than baseline at the study end, painting a confusing picture of bone turnover with androgen treatment .
In muscle, testosterone is well known to cause increases in both muscle volume and strength . Few studies have reported the effects of testosterone on both muscle and bone simultaneously, however. Two studies of transdermal testosterone injections in 67 and 131 men with low bioavailable testosterone found improvements in both bone mass and lean body mass, with an associated decrease in adipose tissue. One of these studies found an increase in lower limb muscle strength, though was confounded by simultaneous vitamin D supplementation , and the other found no change in functional measures . A recent observational study had contrasting findings, showing that free testosterone and its precursor hormone dehydroepiandrosterone (DHEA) correlated to muscle strength and bone architecture in both older men and postmenopausal women . While these studies lend support to testosterone as an anabolic agent in both bone and muscle, the lack of functional and fracture risk data leaves critical unanswered questions.
There is also little information available on the effects of testosterone on women, due to significant adverse effects related to its use, such as acne, male pattern baldness, cardiovascular disease, and potentially breast and gynecological malignancy . At present, there are no widely approved indications for the use of testosterone in women . As osteosarcopenia disproportionately affects women, this is a significant limitation in its use. Other androgenic compounds have been examined as potential candidates for a dual muscle-bone pharmacotherapeutic. The anabolic steroids are synthetic testosterone or androgen analogs that have classically been used in a sports context to enhance muscle strength, mass, and performance. Common anabolic steroids such as nandrolone decanoate have been shown to improve both bone and muscle mass , as well as decrease fracture risk . Unfortunately, due to their nonspecific action on androgen receptors, recipients of anabolic steroids suffer the same issues of widespread testosterone use, making their utilization for osteosarcopenia unlikely.
Selective androgen receptor modulators
In the face of these challenges, research has turned to selective androgen receptor modulators (SARMs) as a method to exploit the anabolic potential of testosterone in osteosarcopenia. The SARMs are ligand compounds that can activate androgen-mediated pathways in some tissues (i.e., bone and muscle) but have less or no impact on others such as the gonads . This occurs by exploitation of the different androgen receptor isoforms expressed in different tissues showing preferential or specific action for the SARM ligands . The perfect SARMs would provide the musculoskeletal anabolic effects of testosterone while having none of the off-target effects. There are a number of SARMs currently being evaluated, with some compounds currently being evaluated in clinical trials.
The SARMs andarine and the closely related ostarine have had the largest body of research behind them. Andarine is commonly described as an ideal SARM, due to its ready bioavailability and long half-life, as well as strong results with few side effects in preclinical studies , though it has principally been studied in the treatment of benign prostate hyperplasia . However, it has more recently been replaced by ostarine as the compound leading the race to clinical utilization in musculoskeletal disease. Ostarine has been shown in a small number of studies to have anabolic effects in muscle and bone, both individually and together. Ostarine has been studied mostly in the treatment of secondary muscle wasting and sarcopenia. In phase II trials, ostarine (under the trade name Enobosarm) has been shown to increase lean body mass and muscle power in older men and postmenopausal women , as well as in cancer patients . Interestingly, this effect appears to happen via multiple pathways, with androgen-independent impacts in satellite cells . Unfortunately, Enobosarm failed to reach its primary endpoints in phase III trials in cancer patients, leading to delays in evaluation . Ostarine has been shown to improve bone mass and architecture in ovariectomized rats, a common animal model of osteoporosis, after 5 weeks of administration . It also improves bone healing after osteotomy in rats, alongside an increase in muscle mass, providing hope for a possible treatment for osteosarcopenia in the future, though currently, no studies have shown positive effects on bone in humans . Though ostarine is the most well-studied compound, a number of other SARMs have also been shown to have benefits in muscle, or are currently being evaluated in trials , though none are currently investigating an effect on bone.
Growth hormone
Given the challenges facing the therapies utilizing androgen stimulation, interest has grown in other endocrine signaling pathways which may have the potential for therapeutic exploitation in osteosarcopenia. Principle among these is growth hormone for its well-defined role in the development of musculoskeletal tissues in puberty and young adulthood. Growth hormone is episodically released from the pituitary gland, with secretion peaking in the second decade. It has a large number of biological roles, but of particular import to osteosarcopenia is its direct stimulation of bone mineralization and muscle growth, via insulin-like growth factor 1 (IGF-1) secretion from the liver. A large protein molecule, growth hormone acts via cell surface membrane receptors in target tissues, with actions mediated by MAPK/ERK, JAK/STAT, and PI3K signaling resulting in specific actions in particular cell types.
Growth hormone is well correlated with musculoskeletal development. Deficiency of growth hormone leads to leads to short stature in children and osteoporosis and muscle wasting in older persons , while excess in early life leads to pituitary gigantism or acromegaly in adults. Growth hormone treatment has been evaluated with respect to effects in both muscle and bone with mixed results. Early studies found promising results, with increases in both bone and muscle mass after 6 months of treatment in older men . However, the studies that followed had more inconsistent results. The evidence for growth hormone as an anabolic approach for muscle is relatively sound, generally finding that it increases muscle mass overall . However, the improvements have tended to be small and have a limited impact on function . In the treatment of bone, growth hormone does not appear to improve BMD, but it may reduce fracture risk in osteoporotic women . Unfortunately, the use of growth hormone as treatment comes with an increased incidence of adverse events, limiting its utility, and diminishing research interest in recent years .
More recently, other agents in the physiological pathway of growth hormone have also been investigated. Ghrelin is a hormone produced by the gastrointestinal tract which stimulates the release of growth hormone. It is commonly referred to as the hunger hormone, as it strongly stimulates appetite in humans, with rising plasma levels rising before eating and falling after. It is presently being evaluated for use in cancer-associated anorexia and cachexia, due to its strong effect on appetite. However, as ghrelin stimulates a native pulsatile release of growth hormone, a hypothesis developed that it may be useful as a bone and muscle anabolic in osteosarcopenic patients. Studies have shown a small positive effect on muscle mass , but with inconsistent functional results. The ghrelin analog anamorelin was refused marketing approval by the European Medicines Agency in 2017, citing limited effectiveness, halting progress on its evaluation. There is a registered clinical trial evaluating the effect of anamorelin on osteosarcopenic patients (trial number: NCT04021706 ), which may shed light on the potential for its clinical use in the future.
Antifracture medications as muscle anabolics
Repurposing medications for osteoporosis for use in osteosarcopenia is an appealing strategy, however few of the approved medications have shown effect on muscle. At present, the only approved medication for osteoporosis with evidence suggesting an improvement in muscle is the monoclonal antibody treatment denosumab. Denosumab is a humanized monoclonal antibody targeting the receptor activator of nuclear factor κ-Β ligand (RANKL), a key factor in the maturation of osteoclasts. It is marketed as Prolia for the treatment of postmenopausal osteoporosis, and as Xgexa for the treatment of malignant bone metastases. Inhibition of RANKL leads to a decrease in the number of active osteoclasts, with an associated decrease in bone resorption. Denosumab has been well-documented to be successful in preventing fractures , and is now in widespread use for the treatment of postmenopausal osteoporosis. New evidence has begun to identify a role for denosumab in the treatment of muscle loss, providing hope for treatment in osteosarcopenia. In the FREEDOM trial on osteoporotic fracture risk with denosumab treatment, it was found that in addition to the expected improvement in fracture rate and BMD, participants receiving denosumab also showed a significant decrease in falls—a hallmark of sarcopenia . This prompted further research into the effect of denosumab on muscle function parameters, with studies finding that RANKL inhibition caused increased muscle strength, as well as insulin sensitivity . Another study of community-dwelling adults taking either zoledronic acid or denosumab found improved gait velocity, balance and agility, and reduced fear of falling in those taking the RANKL inhibitor . This provides some evidence for a role for RANKL inhibitors in the treatment of osteosarcopenia, however, more information is required to validate these findings. It is as yet unclear as to whether these muscle benefits are imparted through changes in lean mass, contractile force, or another unknown mechanism. Future studies on denosumab should include assessment of muscle function and volume in a larger clinical trial.
The crosstalk of muscle and bone as a therapeutic avenue
In recent times, a growing focus has been building on the crosstalk between muscle, bone, and fat . With the discovery of agents that are secreted from one of these tissues that impact the others, a range of new targets for anabolic interventions were identified. One of the key agents in this area is myostatin, a myokine secreted by muscle tissue in response to decreased muscular loading such as inactivity, injury, or sarcopenia. Myostatin acts to diminish muscular differentiation and protein synthesis, in part through Akt signaling , and its exogenous administration causes muscle wasting in mice . Considering these effects, myostatin inhibition has been evaluated as a therapeutic method to increase bone mass.
In animal models, myostatin inhibitors have been shown to cause improvements in muscle mass in models of aging , muscular dystrophy , and cancer-induced cachexia . These compounds have also been evaluated in humans, with a study on older fallers finding improvements in both lean mass and muscle function . Another study of muscular dystrophy found that a myostatin inhibitor improved contractile strength in humans . Generally, the inhibitors have been well-tolerated, with few significant adverse events . However, from the view of osteosarcopenia, their effect on bone is yet to be demonstrated. Myostatin is known to inhibit osteoblast differentiation and osteogenesis , as well as osteoclast formation through RANKL signaling . In mice, inhibition of myostatin has been shown to have anabolic effects on bone. Myostatin deficient mice have been shown to have greater bone mineral content, particularly at sites of muscle attachment , a greater osteogenic response to exercise , and increased bone healing after fracture . A myostatin inhibitor has also been shown to cause an increase in osteogenesis , though another compound tested in the same study improved muscle alone. While positive, effects of myostatin inhibition on bone are yet to be demonstrated, leaving this a hopeful, but an unproven candidate for the treatment of osteosarcopenia.
While myostatin has garnered the most attention as a therapeutic in muscle and bone, a range of other factors has been identified in recent years as having a dual role in both tissues. Compounds such as osteocalcin, irisin, and interleukin-6 have effects on both physiologies, however, no strong data has emerged yet to suggest a role for them as therapeutics .
Future directions and conclusions
Despite recent interest in dual anabolic therapies for muscle and bone in osteosarcopenia, potential therapeutics are still in their infancy. It is clear from recent developments that broad multisystem approaches are required to identify effective solutions. Crosstalk between muscle, bone, and fat is a key part of the pathogenesis of osteosarcopenia, and it stands to reason that consideration of the axes between these tissues will elucidate treatments for the disease . Inhibitors of the lipotoxic effect of fat on muscle and bone, myokine stimulants or inhibitors of osteoclastogenesis, or completely external tissues which play a role in their development and maintenance could provide insight into efficacious methods of their treatment. Additionally, the approved or experimental treatments for osteoporosis or sarcopenia separately should be evaluated for an effect on their counterpart tissue. Their tightly interwoven physiologies of bone and muscle ensure that there is some likelihood of overlap in the therapeutic activity of these compounds, giving opportunity for fast progression in the area. For example, a study has found pamidronate, one of the first nitrogenous bisphosphonates was able to attenuate muscle loss after significant burns in children , however, this has not undergone further investigation to date. Finally, with the advent of cost-effective and efficient approaches for the assessment of genetics and the discovery of methods to manipulate genes safely, methods such as CRISPR may one day allow for targeting specific genetic pathways leading to disease. While still in the far future, as the exponential advance of medical sciences continues, these approaches will no doubt begin to be applied to the aging process in the musculoskeletal system.
While there have been some advances in finding dual muscle and bone pharmacotherapeutic approaches in recent years, there remains a long road ahead. As the focus on the androgen and endocrine pathways begins to recede, identification of new targets must become the priority, alongside evaluation of the promising molecules already identified. Understanding the interplay between the physiologies of bone, muscle and fat should be front and center of research efforts , as it will allow for the identification of the key factors and targets which co-regulate these tissues. These are likely to be the origin for the development of successful therapeutic approaches but will depend on the understanding of their role in health and disease.
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree