Fig. 9.1
Stressors are typically grouped as for physical, metabolic, or psychological origin. Usually, there is a perfect mixture of the three; it is very uncommon to find that only one is acting as unique stressor
These physical, psychological and metabolic conditions are identified as “stressors” and negatively affect GnRH release and the reproductive axis, activating or inhibiting hypothalamic and/or extra-hypothalamic areas in brain as well as acting in the periphery. In particular, one of the key events of this modulatory action is played by neurotransmitters and neuropeptides produced in the central nervous system. These neuronal pathways are sensitive to external and internal environmental changes (light-dark cycle, temperature) as well as to cognitive, social, cultural, and emotional events. Each of these signals may become stressor agents when acute changes occur, and through the integration with the hormonal signals, they can stimulate adaptive responses.
It might be considered strange, but such negative hypothalamic response is nothing else than a defensive system. In primate females, and in human females in particular, an adaptative mechanism during stress is represented by the reduction of reproductive axis activity, blocking a function which is not really essential to survive. Some intermediate steps such as poly- or oligo-menorrhea can anticipate the occurrence of the amenorrheic condition, which is the last and worst stage of this clinical adaptive response to stress. What is interesting to observe is that human species has been going on evolving in these 20 thousand years, especially from the technological and cultural point of view, permitting an incredible change of lifestyle and surviving so that the expectancy of life is incredibly longer. Although with such changes, the endocrine and neuroendocrine systems of the human species have been demonstrated to work and react as they feel to be still frozen 10–15 thousand years ago. In fact, although evolution permitted our species “to come down” from the trees and to start to move as bipeds, no more as simple primates, our neuroendocrine systems apparently work as a primate, as nothing was changed from those times. The physiological meaning of this is extremely important since it indicates that whatever stressor of the twenty-first century (dieting, sport, training, or psychological stressors) hits the human being, the adaptive response is similar and superimposable to the one that might be induced by the sight or the attack of a wild animal or by lack of food or by strenuous fatigue due to a migration.
9.2 Neuroendocrine Mechanisms of Stress-Induced Hypogonadism
Most of the knowledge about stress-induced reproductive failure passes through many experimental animal models. In monkeys and in rats, the common response to stressors is the increase of adrenocorticotropic hormone (ACTH) and cortisol plasma levels. It has been demonstrated that the intraventricular injection of corticotropin-releasing hormone (CRF) reduces GnRH and LH release [7, 8]. Since corticotrophin-releasing hormone (CRF) is the specific hypothalamic stimulating factor for ACTH, elevation of ACTH in response to stress is anticipated by the elevation of CRF stimulation. The evidence of a central site of action for CRF in blocking GnRH-induced LH release is demonstrated by the fact that CRF antagonists reverse the stress-induced LH decrease in rats [7]. CRF elevation as adaptative response to stress is also responsible for the increase of central ß-endorphin (ßEP) release. This last is probably the most important peptide of the endogenous opioid peptides (EOPs) family and is a potent inhibitor of GnRH-LH secretion. Because of this evidence, a connection has been suggested between the activation of the hypothalamus-pituitary-adrenal (HPA) axis and the stress inhibition of the hypothalamus-pituitary-gonadal (HPG) axis [9]. Since naloxone, a specific opioid receptor antagonist, is able to counteract the CRF-induced LH secretory blockade [10], opioid peptides have been considered the key factor of the stress-induced inhibition of the HPG axis.
Peripheral hormonal signals such as glucorticoid hormones or prolactin (PRL) are also activated by stress and are able to act as stress-induced hormonal signal. In fact, cortisol itself exerts a suppressive effect on GnRH-stimulated LH release in rats [11], and such an action is mainly played at the pituitary level, but it cannot be excluded that an additional negative effect may be present on extrapituitary areas, indirectly inhibiting LH secretion [12]. Also prolactin is increased and responds to external stimuli, including emotional and physical events as well as internal rhythms such as sleep. This mechanism has been extensively studied in rats [13] and is mediated by the activation of several stimulating factors like thyrotropin-releasing hormone, vasoactive intestinal peptide, and oxytocin or by the failure of the dopaminergic control. In any case, the final result of stress-related hormone responses is a negative effect both on gonadotropin secretion and on gonadal steroid biosynthesis.
9.3 Neuroendocrine Mechanism of Hypothalamic Amenorrhea
Hypothalamic amenorrhea [14–16] is a model of hypogonadism characterized by several neuroendocrine aberrations, which occur after a relatively long period of time of exposure to a repetitive and/or chronic stressor(s) to affect the neuroendocrine hypothalamic activity [17, 18] as well as the release of several hypophyseal hormones [9, 16, 18–21]. The reproductive axis is severely altered in these patients, and both the opioid and dopaminergic systems have been proposed as potential mediators of stress-related amenorrhea in humans [22, 23].
As demonstrated in experimental animals, the EOPs exert an inhibitory effect on the episodic release of both GnRH and LH also in humans [24, 25], with some differences which are of great interest. When the specific opioid receptor antagonist naloxone is administered, it causes the increase of LH plasma levels during the late follicular and luteal phases of the menstrual cycle but not during the early follicular phase [10, 26, 27]. During postmenopause, the naloxone administration does not induce any LH release, but when patients underwent estradiol administration, LH plasma levels diminished and the naloxone-induced LH response is restored, thus demonstrating that a tight relationship links opioidergic system with gonadal steroids especially estrogens [28] (Fig. 9.2). Also hypothalamic amenorrhea is not responsive to naloxone [29, 30], but after 2 months of hormonal replacement therapy, LH response to naloxone was recovered in at least 53 % of the patients [31].
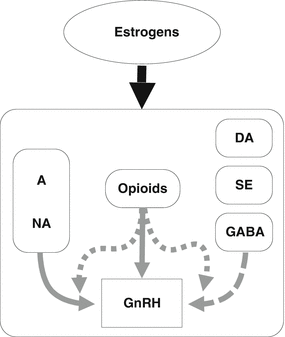

Fig. 9.2
The role of estrogens is essential in maintaining specific neuronal/neuroendocrine functions. When stress induces neuroendocrine dysfunction(s), quite often, a hypoestrogenic condition occurs as a consequence of the ovarian blockade worsening the stress-induced abnormal neuroendocrine control of GnRH release from the hypothalamus
On this basis, several studies have been done on the efficacy of opioid receptor antagonist on the restoration of a normal gonadotropin release and of menstrual cyclicity [31–33]. These data seem to confirm that in stress-induced amenorrhea, opioidergic system can be antagonized by the chronic administration of naltrexone, an opioid receptor antagonist, independently from the body weight recovery. This last appears to be influenced by naltrexone centrally and/or by the increase of gonadal steroid plasma levels as soon as the menstrual cyclicity is restored, thus confirming that opioidergic tone and/or steroid milieu is deeply involved in the modulation of the many central nervous system functions/activities such as the hypothalamic centers ruling food intake.
Also the dopaminergic system is involved in the negative modulation of GnRH release. In fact, the blockade of dopamine receptors by metoclopramide caused the increase in LH plasma levels in women with hypothalamic amenorrhea but not in eumenorrheic women [22], thus supporting the evidence of an impaired dopaminergic activity in stress-induced amenorrhea.
Impaired serotoninergic activity has been reported in hypothalamic amenorrhea. In fact, a blunted cortisol response to fenfluramine, a serotoninergic agonist, has been demonstrated and related to an increased serotoninergic tone [34, 35]. Serotonin seems to participate to the stress-induced neuroendocrine events leading to the HPA axis impaired activity and interacts with EOPs in the regulation of the spontaneous GnRH-induced LH release. This interaction between the opioidergic and serotoninergic axes is supported by the fact that in normal subjects, fenluramine administration blocks the naloxone-induced LH secretion and determines the significant increase of plasma ACTH and cortisol plasma levels [34, 35]. However, even though the administration of cyproheptdine cloridrate, a serotonin receptor antagonist, at the dose of 4 mg/day has been demonstrated to be effective in increasing LH, FSH, GH, and fT3 plasma levels, no effect of cyproheptadine was observed on naloxone-induced LH response in patients with HA [36].
9.4 Metabolic Signals as Stressors in Hypothalamic Amenorrhea
The mechanisms by which undernutrition and/or energy consumption causes the decrease in central drive to the reproductive axis are complex and also probably involve not yet known pathways. However, a clear correlation exists between the incidence of reproductive dysfunction and the severity of body weight loss [2, 37]. As mentioned above, the percentage of body fat lost seems to be a determinant for the induction of the blockade of the reproductive axis. In fact, the suppression of the central drive(s) to the HPG axis in undernutrition takes place only when the lost of weight includes a significant loss of body fat [37, 38]. An alternative hypothesis is that in the early stages of dieting or undernutrition, some physiological changes serve as signal to activate the suppression of the reproductive axis, and the more is the undernutrition state, the more profound the block becomes, leading to a greater suppression of the HPG axis [5]. Among the signals that can trigger specific defensive hypothalamic behavior, there are both the low insulin and fT3 plasma levels [36]. The decreased fT3 levels are defined as “low T3 syndrome,” and it occurs to protect the excessive consumption of energy for heating purposes. A higher amount of the biologically inactive reverse-T3 (rT3) is synthesized from fT4 deiodation, without any change on the feedback linking TSH and thyroid hormones [2, 36].
The efficacy of dieting in inducing a specific central change is supported by several data on primates. One day after initiation of fasting, monkeys show a significant reduction of gonadotropin episodic discharge with the concomitant reduction of mean plasma concentrations of LH, FSH, and gonadal steroids [39]. Fasting-induced suppression of gonadotropin release results from a decrease in hypothalamic stimulation to the pituitary rather than from a decrease in pituitary responsiveness to endogenous GnRH [39]. Similar results were observed in humans [40], and it is of interest to observe that in hypothalamic amenorrhea, LH more than FSH seems to be the more sensitive to the hypothalamus functional impairment, since only LH plasma levels are significantly reduced [16, 19, 41]. These data have also enforced the possible existence of an additional system(s) modulating FSH but not LH episodic release from pituitary in humans [42, 43] (Fig. 9.3).
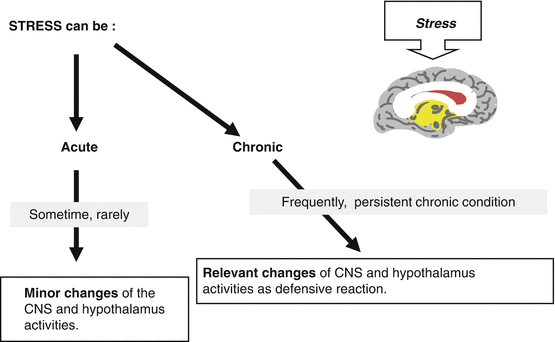

Fig. 9.3
Stress-induced neuroendocrine malfunction occurs only when such stressors act in a repetitive (chronic) manner
9.5 Clinical Considerations of Stress-Induced Reproductive Impairment
All the above-discussed items are extremely important since they give us the clear idea that hypothalamus in females is a great “computer” that takes care of all inputs and defines specific outputs to monitor/modulate/regulate a lot of other biological functions such as sleep, glucose control, heart function, feeding, reproduction, and many others. This happens also in males, but it is incredibly active and sensitive in females. Try to think for a moment as if a human female is living, let us say 20 thousand years ago. For that time, reproduction was probably the less important function since a human being (or a primate) could perfectly survive without reproduction and the survival of the single human being could not be guaranteed if reproduction hits its goal (i.e., the occurrence of a pregnancy) especially during seasons/periods during which no food was available or a strenuous energy consumption was ongoing (migration), as well as psychological stressors (fear to die). For those times, a pregnancy in the wrong moment might mean an incredibly higher risk to die. To avoid this and to avoid losing that single human being, the hypothalamus developed the ability to block the reproductive axis in reversible way to avoid ovarian function (i.e., ovulation), with the chance to restart it only when the external conditions might be improved and more adequate.
Life in this twenty-first century is not as that many thousands years ago, but there are always a lot of stressful events that can trigger our hypothalamus and omeostatic system(s) and induce a blockade of reproduction. Although this has to be considered an incredibly well-designed defensive system, unfortunately, it might be activated in peculiar period of times such as during pubertal development, inducing a delayed menarche/puberty. Indeed, when intensive sport training or recreational activity is started at a prepubertal age, it is not so rare to observe a significant delay in the occurrence of pubertal maturation up to 2–4 years [3, 44]. More or less, there is a delay of 5 months in the occurrence of menarche for each year of training [3]. Such observation resulted evidently in prepubertal/pubertal girls performing sport and dance training [3, 14, 44] and in young postmenarchal adolescents who reported the occurrence of amenorrhea. The meaning of this observation is quite obvious since it indicates that if energy consumption and physical stressors are too much, growth and reproductive axis maturation might not occur in the same time. In fact, most of the girls who suffer from this condition recover into normal development and/or menstrual cyclicity as soon as training is significantly reduced or suspended. The presence of the right amount of fat is fundamental for pubertal development since it is considered as a specific and obliged reserve of energy that the body might need to have stored in case a pregnancy occurs within the very first ovarian cycles [37, 44]. Nowadays, this reserve of fat is important just for the beginning of the menstrual cyclicity (i.e., menarche) since pregnancy during pubertal age is not so common in Western countries (more frequent in underdeveloped countries), but it is important to consider this biological aspect every time we study a girl who wants to start or has just started any kind of physical activity. Physical activity has to not be strenuous or stressful to avoid any conflict with the maturation/activation of her reproductive axis. A delay in such event might induce reduced bone mass peak, osteopenia, and then reduced bone mass density during fertile life, exposing the girl to damages of the skeleton and of the muscle activity. Hypoestrogenism during adolescence, with no menarche and/or menstrual cyclicity and with a sub-normal development of sex-steroid-dependent tissues (fat distribution, breast development), might be responsible also for an abnormal or conflicting self-image.
9.6 Putative Treatments for Hypothalamic Amenorrhea
It results quite clear that the scenario of the physiopathological factors that trigger the occurrence of the hypothalamus-pituitary blockade is quite large and that most of the factors involved are tightly interconnected with each other. On the basis of what was previously known and on what our group investigated in the recent years, a sort of schematical and putative list of therapeutic actions can be proposed, as listed in Table 9.1.
Table 9.1
Putative therapeutical strategies for stress-induced hypothalamic amenorrhea have to act on one or more of the following
CNS peptides (opioids, CRF) |
Neurotransmitters, neuromodulators (DA, GABA, serotonin, etc.) |
Various hormones (PRL, thyroid hormones) |
Low-dose estrogenic priming |
Feeding and/or energy balance (proteins, glucose, training) |
Although schematisms might suggest that things are much easier than what the reality is, this chapter will try to give some therapeutical advice to help in choosing the therapeutical approach/strategy according to the area on which it has to act.
9.6.1 CNS Peptides (Opioids, CRF)
As previously described, the hypothalamic nuclei that secrete GnRH are modulated by a large variety of peptide, neuropeptides and amines. In these last decades, various studies have clearly demonstrated that stress induces a higher production of endogenous opioids (mainly beta-endorphin) that negatively affect GnRH production and discharge. Moreover, stress induced by dieting and/or excessive energy expenditure, such as in athletes and dancers, triggers similar neuropeptides with the addition of the negative modulation induced by the lack of energy availability (i.e., reduced fat mass, feeding using low caloric diet). In such conditions, it has been reported that acetyl-L-carnitine (ALC) administration up to 1 g every day is effective in inducing the recovery of the endogenous as well as the exogenous GnRH-induced LH secretion [45] and the restoration of the normal response to naloxone infusion [46]. It has been suggested that ALC has a pivotal role in the transport of activated fatty acids for β-oxidation to produce energy in the mitochondria, especially in dieting or impaired energy metabolism [45, 46]. In addition, acetyl groups derived from ALC are used inside the brain for inactivation of the amines and neuropeptides (such as opioids) produced in higher amount for the stress [46]. Similarly to ALC, naltrexone cloridrate administration actively counteracts the negative modulation exerted by the stress-induced hyperproduction of opioidergic compounds, mainly β-endorphin [31–33]. Indeed, it has been demonstrated that in hypothalamic amenorrhea, naltrexone administration, a long-acting opioid receptor antagonist, is effective in restoring the hypothalamic GnRH discharge and ovulatory cycles in hypothalamic amenorrhea associated with weight loss [32], independently from the response observed to naloxone infusion before starting treatment [33]. This last study reported that the recovery of the ovarian function occurred in 80 % of the patients and resulted to be independent from the body weight recovery, which appeared exactly 3 months after the menstrual cyclicity was recovered [33]. According to preliminary data from our group, slightly higher clinical improvement has been observed when ALC and naltrexone cloridrate are administered together.
9.6.2 Neurotransmitters, Neuromodulators (DA, GABA, Serotonin, etc.)
Among the mediators of the opiatergic and/or dopaminergic systems [22, 23] in HA, a role has been proposed for gamma-aminobutyric acid (GABA), a specific modulator of the physiological response to stress or on anxiety [47]. In fact, various acute and chronic stressors have been demonstrated to produce a rapid decrease of the activity of GABAergic pathways in primates and in humans [48, 49]. The fact that stress and anxiety stimulate both the secretion of corticotropin-releasing factor (CRF) and modulate GABAergic neurons suggests a possible functional interaction between these two systems. Indeed, GABAergic or benzodiazepine receptor-mediated mechanisms inhibit CRF release [50], and anxiolytic benzodiazepines can reverse or antagonize in experimental animals several CRF-mediated behavioral effects that are thought to be related to stress [51, 52].
A neurotropic compound, pivagabine (PVG), a hydrophobic-4 aminobutyric acid derivative [53], has been shown to exert specific effects on stress-induced activities in rats [53, 54]. Experimental data showed also specific inhibitory actions of PVG on some behavioral parameters in rats exposed to various stressors [55], probably acting indirectly on GABA receptor type A (GABAA) [48, 49]. Since PVG prevents the reduction of hypothalamic contents of CRF and its discharge from hypothalamic neurons [56] in rats, it has been supposed that PVG might modulate the adaptative response to stress. When PVG was administered to a group of patients with HA-specific modulation of GH, ACTH, and cortisol secretions were observed [57]. The reduction of cortisol plasma levels is a clear marker of the neuroendocrine modulation exerted by PVG, since patients with HA have usually cortisol plasma levels at the higher level from the normal range. These data sustained the role of anti-stress activity of PVG on patients with highly activated HPA axis [57, 58] and quite often in the presence of a disturbed metabolic balance [59], being all of them causal factors of the reproductive failure of these patients. In addition, PVG has been reported to reduce anxiety and depression in postmenopausal women [60]. Probably PVG modulates the release of hypothalamic CRF and/or pituitary ACTH, and this last observation is in agreement with the fact that the increased GH release might also be related to the PVG-induced decrease of CRF hypothalamic tone. In fact, CRF is involved in GH regulation, and acute CRF administration inhibited GHRH-induced GH secretion probably though a higher somatostatin release [57, 61].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree