PK parameter
Dimension
Relevance
t 1/2zp
Time
Transfer from blood to deep compartment
t 1/2el
Time
Elimination half-life from the body
c max
Concentration/volume
Peak concentration in blood or tissue
t max
time
Time to reach c max
AUC
Concentration/volume × time
Area under concentration-time curve
Cltot
Volume/time
Total body clearance
Vd
Volume
Volume of distribution
The concentration of a drug in the target organ can be increased by using special applications such as regional drug administration. By changing the actual physiological conditions of the target organ (for instance, by occlusion of a blood vessel), regional administration increases the absorption rate of the chemotherapeutic agent from the blood into the tumor tissue. As a consequence, blood flow is decreased through the affected organ, and tissue-extraction rate is accelerated or increased.
So regional administration combined with a temporary occlusion of the supplying vessels is a valuable therapeutic option, especially for the chemotherapeutic treatment of liver tumors and liver metastases, respectively.
2.2 Hepatic Blood Flow (Q hep)
The perfusion of the liver is a main factor of the regional administration. Hepatic blood flow is the sum of portal vein (1,050 ml/min) and common hepatic artery (300 ml/min) blood flow. Therefore, Q hep is about 1,500 ml/min (≈90 l/h).
2.3 Hepatic Extraction Rate (E hep)
E hep is calculated as follows by the arterial and venous drug concentration during liver passage.
 E hep ranges from 0.0 (=no extraction) to 1.0 (=complete extraction). An E hep of 0.8 indicates the elimination and metabolism of 80 % of the drug entering the liver leaving 20 % of the administered drug to exit the liver through the liver veins.
E hep ranges from 0.0 (=no extraction) to 1.0 (=complete extraction). An E hep of 0.8 indicates the elimination and metabolism of 80 % of the drug entering the liver leaving 20 % of the administered drug to exit the liver through the liver veins.

2.4 Hepatic Clearance (Clhep)
Clhep is defined as the volume of blood passing through the liver that is cleared from a compound per time. Hepatic clearance is based on the whole body clearance minus the renal clearance and the mostly quantitative not relevant non-hepatic, nonrenal clearance by other organs (e.g., skin or lung). Clhep depends on the blood flow through the liver, the liver cell mass, and the activity of drug-metabolizing enzymes. It is the product of E hep and the blood flow through the organ (Q hep).
 Considering the hepatic extraction of a drug, its tissue penetration does not only depend on physiological conditions (as already mentioned) but also on the physicochemical properties of the molecule as well. Besides the drug there are some other factors with impact on the hepatic clearance (see Table 2.2).
Considering the hepatic extraction of a drug, its tissue penetration does not only depend on physiological conditions (as already mentioned) but also on the physicochemical properties of the molecule as well. Besides the drug there are some other factors with impact on the hepatic clearance (see Table 2.2).

Table 2.2
Factors that have an influence on E hep of a drug
Parameter | Mechanism |
|---|---|
Blood flow | Distribution rate |
Tissue uptake | Absorption mechanism (diffusion, active transport) |
Protein binding | Intravascular depot |
Liver diseases | Altered vascularization, dysproteinemia |
Cytostatic | Physicochemical properties (lipophilicity, pka value, ionization) metabolism (phases I and II) |
Occlusion method | Means and duration of occlusion, amount of particles |
Despite their chemical heterogeneity, a number of different cytostatic agents can be used for regional intraarterial treatment (see Table 2.3). The most important assumption for the drug is a so-called first-pass metabolism or first-pass effect. Per definition first-pass effect is the sum of all processes (distribution and metabolism) occurring during the first liver passage of a drug before the drug reaches systemic blood circulation and becomes available in the whole body.
Drug | Vd (l) | Cltot (l/min) | t 1/2 (h) | Metabolism |
|---|---|---|---|---|
Doxorubicin | ≈1.500 | 1.2 | 30 | Liver |
Epirubicin | ≈2.000 | 1.2 | 35 | Liver |
5-Fluorouracil | 16 | 2.0 | 0.3 | Ana-, catabolism |
Irinotecan | 200–400 | 0.5 | 15 | Liver |
Mitomycin C | ≈50 | 1.1 | 0.6 | Blood metabolites |
Pt-agents | 30 (UF) | 0.04 | 150 | Blood metabonates |
Gemcitabine | 85 | 0.8–1.5 | 0.5–1.5 | Liver, leucocytes |
Carmustine | 250 | ≈4.2 | 1.5 | Metabonates |
By comparing the intraarterial/intravenous AUC ratio, chemoembolization leads to a therapeutic advantage (TA), calculated as follows:
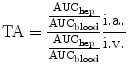 In comparison to IV administration, decreasing hepatic perfusion results in a higher regional distribution rate.
In comparison to IV administration, decreasing hepatic perfusion results in a higher regional distribution rate.
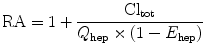 Regional application combines decreasing side effects and higher levels of toxicity (increased apoptosis rate) [4]. The RA gets more intense the faster the cytostatic distributes into the tissue and the higher its extraction rate from the body.
Regional application combines decreasing side effects and higher levels of toxicity (increased apoptosis rate) [4]. The RA gets more intense the faster the cytostatic distributes into the tissue and the higher its extraction rate from the body.
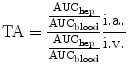
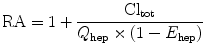
2.5 Pharmacokinetic Data Using Degradable Starch Microspheres (DSM)
A successful embolization can be characterized by comparing the main pharmacokinetic parameters with data obtained after conventional administration. AUClast and c max are the most suitable values for calculating the shift of the drug’s concentration from blood to tissue.
Depending on the chemotherapeutic agent, the administration of DSM leads to a decrease of systemic circulation from 20 to 60 %. It is the most important requirement that the chemotherapeutic does not bind to DSM or red blood cells [5].
So far most of the studies concerning pharmacokinetic data of cytostatic agents after the embolization of the common hepatic artery used DSM. The findings in Table 2.4 from several studies show between 19 and 98 % reductions in plasma drug concentrations. The reduced systemic drug exposure may be seen as an increased first-pass extraction during the prolonged time of the drug in the occluded target area. The higher first-pass extraction of the drug in the target compartment will lead to a lower dose of drug reaching the systemic circulation and subsequently to fewer side effects [6, 14]. Besides the chemotherapeutics given in Table 2.4, one of the most currently irinotecan is administered intraarterially after chemoembolization as well [19]. Irinotecan (CPT-11) is a prodrug and needs to be activated in the body. The drug shows poor affinity to the responsible enzyme (human carboxylesterase); therefore, only small amounts of the pharmacologic active metabolite SN-38 are formed (about 10 % of the parent compound). This activation can be improved by regional administration to the liver leading to higher amounts of SN-38 in the blood and tissue.
Table 2.4
Mean reduction of plasma AUC in patients with HCC using DSM
Drug | Tumor type | AUC decrease (%) | N | References |
|---|---|---|---|---|
Mitomycin C | Primary and secondary liver cancer | 33 | 87 | [6] |
[7] | ||||
[8] | ||||
[9] | ||||
[10] | ||||
[11] | ||||
Doxorubicin | Primary and secondary liver cancer | 19 | 5 | [12] |
[13] | ||||
Carmustine(BCNU) | Primary and secondary liver cancer | 62 | 5 | [14] |
Fotemustine | Primary and secondary liver cancer | 53 | 4 | [15] |
5-FU | Primary and secondary liver cancer | 38 | 8 | [16] |
Floxuridine | Colorectal liver metastasis | 34 | 3 | [10] |
Cisplatin | Colorectal liver metastasis | 38 | 4 | [17] |
Cisplatin andsodium thiosulfate | Head and neck cancer | 98 | 6 | [18] |
Numerous investigations characterized the combination of mitomycin C (MMC) with different amount of DSM. The AUC ratio is relatively consistent from 0.55 to 0.80 as can be seen in Table 2.5. Administration of 60 mg DSM did not show any effect; obviously this amount was too low for any occlusion of blood vessels.
Table 2.5
Average AUC ratio, measured as peripheral plasma AUC of MMC with and without DSM in patients with HCC
DSM (mg) | MMC (mg/m2) | N | AUC ratio | 95 % CI | References |
|---|---|---|---|---|---|
360 | 15 | 36 | 0.74 | 0.62–0.87 | [6] |
360 | 10 | 6 | 0.70 | 0.55–0.88 | [7] |
[9] | |||||
900 | 5–10 | 11 | 0.61 | 0.47–0.80 | [7] |
[9] | |||||
540 | 3 | 7 | 0.73 | 0.62–0.86 | [9] |
900 | 9 | 10 | 0.55 | n.s. | [8] |
360 | 10 | 3 | 0.80 | n.s. | [10] |
450–900 | 18 | 14 | 0.55 | n.s. | [11] |
60 | 20 | 7 | No effect | n.s. | [20] |
More data about the distribution of other cytostatic agents into tumor and healthy tissue using DSM in animals and patients are in Tables 2.6 and 2.7. Table 2.6 gives an overview of experimental findings in animals.
Table 2.6




Ratio of cytostatic drugs in tumor and healthy liver tissue (with and without DSM) in vivo (rat, rabbit)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree