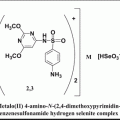
Fig. 14.1
Percentage apoptosis in primary ovarian cancer cells on treatment with metformin and chemotherapeutic agents
In this study, we investigated whether metformin could induce apoptosis in ovarian cancer cells. Metformin has been shown to be selectively toxic to p53-deficient cells and provides a potential mechanism for the reduced incidence of tumors observed in patients being treated with metformin. However, paclitaxel-induced cell death is not dependent on the p53 status of the cells. We found that metformin together with standard chemotherapy was effective in inducing apoptosis in ovarian cancer cells. The wide variation reported between individuals on metformin treatment could perhaps be due to existing polymorphism of the metformin transporter, OCT 1. Taken together, our results suggest that there was an additive effect of carboplatin and metformin on ovarian cancer cells, thus demonstrating a chemoadjuvant potential for EOC, with a combination of metformin, carboplatin, and paclitaxel being the best combination which gave the highest percentage of apoptosis. Metformin is a long-approved drug with good safety record, so clinical tests of these preclinical observations are practical and of potential medical significance. The above observations provide further evidence for an experimental rationale for using metformin as part of a combinational therapy.
In another set of experiments, the cells were treated with curcumin, carboplatin, and paclitaxel alone or in their combinations. The mean percentage of apoptosis in untreated control cells was 5.9 % (ranging from 4.98 % to 6.67 %); for curcumin by itself, it was 12.6 % (range 10–16 %); with carboplatin alone, it was 13.7 % (range 6–25 %); and with paclitaxel, it was 14.8 % (range 7.6–22.6 %). A combination of curcumin and carboplatin increased the mean percentage of apoptosis to 16.5 % (range 7.2–29 %); with curcumin and paclitaxel, it was 24 % (range 20–33 %); and with carboplatin and paclitaxel, it was 22 % (range 15–32 %). However, the addition of curcumin to this combination increased the percentage of apoptotic cells to 45 % (range 32–53.5 %), shown in Fig. 14.2. The findings indicate that curcumin by itself was as good as carboplatin or paclitaxel in inducing apoptosis in primary epithelial ovarian cancer cells and was able to affect the carboplatin-mediated cell killing marginally. However, a combination of curcumin and paclitaxel was additive and was equally effective as the combination of carboplatin and paclitaxel. A combination of curcumin, carboplatin, and paclitaxel was found to be additive and gave the highest percentage of apoptosis.
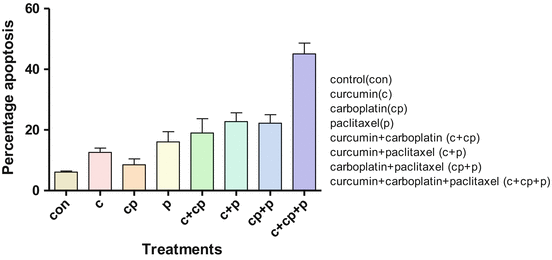

Fig. 14.2
Percentage apoptosis in primary ovarian cancer cells on treatment with curcumin and chemotherapeutic agents
Curcumin has been shown to induce apoptosis in ovarian cancer cell lines with p53 and Bcl-2 family playing an important role in curcumin-mediated apoptosis. Since the primary cultures we established expressed mutated p53, curcumin was not able to affect much of the carboplatin-mediated apoptosis in these cells. On the other hand, paclitaxel inhibits cancer cell growth by interacting with microtubules. It results in cell cycle arrest at G2/M phase followed by apoptosis. Thus, there is a scope for the development of patient-tailored therapy based on molecular profile of the tumor and response of the primary culture cells to various chemotherapeutic agents in vitro.
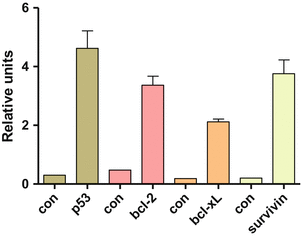
The expression of apoptosis-related proteins was analyzed by Western blotting, shown in Fig. 14.3. The mean expression of P53 in controls was 0.3 RU, whereas it was 4.6 RU in ascitic cells which was significantly higher. The mean expression of Bcl2 was 0.48 RU in controls and 3.36 RU in ascitic. BclXl was 0.19 RU in controls and 2.12 RU in ascitic cells. Similarly, survivin was 0.2 RU in controls and 3.76 RU in ascitis. P53 showed a positive correlation with Bcl2, BclXl, and survivin. Similarly, survivin also positively correlated with Bcl2 and BclXl expression. There was increase in expression of P53, Bcl2, BclXl, and survivin in ascitic cells. Thus, studying ascitic cells of EOC patients may help to delineate the molecular profile of the primary tumor before initiation of therapy and also hold immense potential to be used as a diagnostic tool. It is hypothesized that P53 and Bcl2 family play an important role in curcumin-mediated apoptosis. In contrast, curcumin was not able to enhance much the carboplatin-mediated cell killing in our study. We hypothesize that curcumin is able to enhance carboplatin-mediated cell killing only if the cells express wild-type P53. Since the primary cultures we established expressed mutated P53, curcumin was not able to affect carboplatin-mediated apoptosis in these cells. On the other hand, paclitaxel inhibits cancer cell growth by interacting with microtubules. It results in cell cycle arrest at G2/M phase followed by apoptosis. Paclitaxel-induced cell death is not dependent on the p53 status of the cells. However, increased expression of survivin has been shown to inhibit apoptosis induced by paclitaxel. Survivin is expressed in G2/M phase of the cell cycle and associates with microtubules, preventing their stabilization by paclitaxel. Curcumin is able to downregulate survivin and thus increase sensitivity of cancer cells to paclitaxel. In our study, curcumin was able to enhance paclitaxel-induced cell death in primary cultures, all of which overexpressed survivin. Though curcumin also downregulated BCl2 and increased chemosensitivity of EOC cells, this probably did not play a role as the downregulation of Bcl2 is brought about by wild-type p53.