Fig. 40.1
New types of peptide-based vaccines: Examples of new types of peptide-based vaccines are shown. Gray and black boxes indicate CTL and helper T-cell epitopes, respectively
40.2 No Impact of Recent Advances of Cancer Immunotherapy for aPC
Remarkable advances have been made in the field of cancer immunotherapy in the past 5 years. Immune checkpoint blockers (ICB) (e.g., anti-CTLA-4, anti-PD-1, and anti-PDL-1 antibodies) can achieve durable clinical responses in at least one-fifth of patients with several types of advanced cancer [8–10]. However, these ICB have exhibited very limited clinical benefit for the other cancers, including pancreatic cancer, which displayed no or few tumor-infiltrating lymphocytes [11–13]. Cancer vaccines tested in the past two decades also failed to show clinical benefits for pancreatic cancers [14–16]. In addition to the dearth of tumor-infiltrating lymphocytes, the large heterogeneity of tumor-associated antigens and the diversity in both HLA and T-cell subsets may hamper the successful development of a cancer vaccine for aPC [3–5].
40.3 Rationale for Personalized Selections of Vaccine Peptides
Cancer patients possess antitumor immunity, which may depend strongly on both the tumor cell characteristics and the immunological status of the host [17–20]. The antitumor immunity differs widely among individuals, since the tumor cell characteristics and the host immune cell repertoires are quite diverse and heterogeneous among patients, even among those with identical HLA types and the same pathological types of cancer. Considering the complexity and diversity of the host immune cell repertoires, it is likely that vaccine antigens that are selected and administered without considering the host immunological status would not efficiently induce beneficial antitumor immune responses [20]. To evaluate the host immune cell repertoires, we examined patients’ pre-existing immunity to a panel of vaccine candidates before vaccination and selected appropriate vaccine antigens for which the individual patients exhibited immunological memory [3–5]. Vaccine antigens to which patients already possess antigen-specific immunological memory are expected to elicit quick and strong secondary immune responses after vaccination (Fig. 40.2) [3–5]. In light of this, it would be quite reasonable to select vaccine antigens on the basis of the pre-existing immune cell repertoires in each patient. These facts suggest that our new concept of “personalized” cancer vaccine formulation may confer several advantages, including the possibility of bypassing both immunological diversity and tumor heterogeneity.
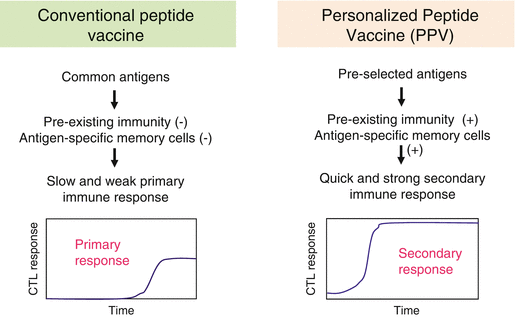

Fig. 40.2
Rationale of personalized peptide vaccine: In conventional peptide vaccines without measuring pre-existing immunity, patients without immunological memory to vaccine antigens would be expected to take more time to develop effective antitumor immune responses, since several rounds of repeated vaccinations might be required to prime antigen-specific naïve T cells to functional effector cells. In personalized peptide vaccines based on pre-existing immunity, patients with antigen-specific immunological memory are expected to show quick and strong secondary immune responses to the selected peptides (This figure is reproduced from reference Sasada et al. [3])
40.4 PPV Procedures
For PPV, a maximum of four peptides are selected based on the results of HLA typing and the pre-existing immune responses specific to each of the 31 HLA class I-restricted cytotoxic T-lymphocyte (CTL) epitope peptides (9-mer or 10-mer short peptides): 12 peptides for HLA-A2, 14 peptides for HLA-A24, 9 peptides for the HLA-A3 supertypes (A3, A11, A31, or A33), and 4 peptides for HLA-A26 (Table 40.1). These peptides were identified mainly through the cDNA expression cloning method with tumor-infiltrating T-lymphocyte lines [3–5, 21–24]. The safety and potential immunological effects of these vaccine candidates have been demonstrated in clinical studies [3–5, 25, 26]. It should be noted that we currently employ these 31 CTL epitopes, which has also been shown to induce antigen-specific B-cell immune responses, as vaccine antigen candidates for PPV, since it has been suggested that a CTL peptide with the ability to induce antigen-specific B-cell responses could provide more effective immune responses than a CTL peptide without this ability [27, 28].
Table 40.1
Peptide candidates for personalized peptide vaccination
Symbol for peptide | HLA type | Origin protein | Position of peptide | Amino acid sequence |
|---|---|---|---|---|
CypB-129 | A2,A3sup | Cyclophilin B | 129–138 | KLKHYGPGWV |
Lck-246 | A2 | p56 lck | 246–254 | KLVERLGAA |
Lck-422 | A2,A3sup | p56 lck | 422–430 | DVWSFGILL |
MAP-432 | A2,A26 | ppMAPkkk | 432–440 | DLLSHAFFA |
WHSC2-103 | A2,A3sup,A26 | WHSC2 | 103–111 | ASLDSDPWV |
HNRPL-501 | A2,A26 | HNRPL | 501–510 | NVLHFFNAPL |
UBE-43 | A2 | UBE2V | 43–51 | RLQEWCSVI |
UBE-85 | A2 | UBE2V | 85–93 | LIADFLSGL |
WHSC2-141 | A2 | WHSC2 | 141–149 | ILGELREKV |
HNRPL-140 | A2 | HNRPL | 140–148 | ALVEFEDVL |
SART3-302 | A2 | SART3 | 302–310 | LLQAEAPRL |
SART3-309 | A2 | SART3 | 309–317 | RLAEYQAYI |
SART2-93 | A24 | SART2 | 93–101 | DYSARWNEI |
SART3-109 | A24,A3sup,A26 | SART3 | 109–118 | VYDYNCHVDL |
Lck-208 | A24 | p56 lck | 208–216 | HYTNASDGL |
PAP-213 | A24 | PAP | 213–221 | LYCESVHNF |
PSA-248 | A24 | PSA | 248–257 | HYRKWIKDTI |
EGFR-800 | A24 | EGFR | 800–809 | DYVREHKDNI |
MRP3-503 | A24 | MRP3 | 503–511 | LYAWEPSFL |
MRP3-1293 | A24 | MRP3 | 1293–1302 | NYSVRYRPGL |
SART2-161 | A24 | SART2 | 161–169 | AYDFLYNYL |
Lck-486 | A24 | p56 lck | 486–494 | TFDYLRSVL |
Lck-488 | A24 | p56 lck | 488–497 | DYLRSVLEDF |
PSMA-624 | A24 | PSMA | 624–632 | TYSVSFDSL |
EZH2-735 | A24 | EZH2 | 735–743 | KYVGIEREM |
PTHrP-102 | A24 | PTHrP | 102–111 | RYLTQETNKV |
SART3-511 | A3sup | SART3 | 511–519 | WLEYYNLER |
SART3-734 | A3sup | SART3 | 734–742 | QIRPIFSNR |
Lck-90 | A3sup | p56 lck | 90–99 | ILEQSGEWWK |
Lck-449 | A3sup | p56 lck | 449–458 | VIQNLERGYR |
PAP-248 | A3sup | PAP | 248–257 | GIHKQKEKSR |
For the selection of peptides suitable for each patient, in the earlier stage of translational studies of PPV, pre-existing immunity was defined by the frequencies of CTL precursors in pre-vaccination peripheral blood mononuclear cells (PBMCs) [29–33]. However, we are currently evaluating the pre-existing immunity to vaccine candidates by measuring peptide-specific IgG titers in pre-vaccination plasma by the multiplex bead-based Luminex assay rather than CTL precursor frequencies, since the performance characteristics, such as the sensitivity and reproducibility, of the current T-cell assays are sometimes unsatisfactory for detecting low frequencies of antigen-specific CTLs [34, 35].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree