Fig. 23.1
Historical evolution of treatment of hypothyroidism
A number of studies, following observations about the benefit of T4 and T3 combination therapy, have largely failed to confirm an advantage of this approach to improve cognitive or mood outcomes in hypothyroid individuals treated with T4 alone [7]. Furthermore, there is no evidence to support using desiccated thyroid hormone in preference to LT4 monotherapy and therefore it should not be used for the treatment of hypothyroidism [8]. Finally, patients taking dietary supplements and nutraceuticals for hypothyroidism should be advised that commercially available thyroid-enhancing products are not a remedy for hypothyroidism and should be counseled about the potential side effects of various preparations, particularly those containing iodine or sympathomimetic amines as well as those marked as “thyroid support” since they could be adulterated with large amounts of thyroid hormones. However, it cannot be excluded that in the future the development of new more effective thyroid hormone analogues or new insights about the individual T4 and T3 requirement will modify the therapeutic strategy in some special populations such as patients with polymorphisms of type 2 deiodinase.
Different formulations of LT4 are currently available and their bioequivalence is still debated. In particular there are solid tablets, soft gel capsules, and oral solutions in drops or monodose vials. The soft gel capsules, recently available in a wide range of strengths, markedly increase the compliance for the patients. Furthermore, liquid oral formulations (drops, vials, and more handy soft gel capsules) offer advantages in patients with difficult swallowing, such as children and elderly patients, and conditions interfering with LT4 absorption (Table 23.1) [9].
Table 23.1
Principal causes of altered absorption of LT4
Transient | Different bioequivalence of LT4 |
Poor compliance | |
Interfering drugs | |
Helicobacter pylori | |
Parasitosis | |
Permanent | Atrophic gastritis |
Celiac disease | |
Lactose intolerance | |
Inflammatory bowel diseases | |
Gastroparesis | |
Short bowel syndrome | |
Gastrointestinal surgery |
In fact oral absorption of drugs from solid tablets is dependent upon the release of the drug from the formulation, the dissolution of the drug, and the permeability of the drug across the gastrointestinal tract. In particular the dissolution is pH sensitive with a higher T4 dose requirement in patients with higher gastric pH. Soft gel capsules containing T4 dissolved in glycerin show the most consistent dissolution pattern and the less pH dependency. This is particularly useful in patients concurrently on T4 and gastric pH altering drugs, such as proton pump inhibitors.
It is worthwhile to note that 6–8 weeks after the formulation or brand of LT4 has been changed A check of thyroid function tests is appropriate, because there may be subtle differences in bioavailability of T4 formulations.
The current approach to calculate the starting dose of LT4 after total thyroidectomy is mainly based on the patient’s body weight (BW). In patients who do not present with cardiac symptoms or malabsorption, the most common recommended starting dose of LT4 ranges from 1.6 to 1.7 μg/kg/day [3, 6]. Since it is an acute hypothyroidism, a gradual titration of the dosage is not needed. However, the mean substitutive LT4 dosage in adults can vary between 1 and 2.2 or more μg/kg per day depending on the type of intervention and possible individual malabsorption. These values are obviously physiologically greater during childhood.
Reaching the optimal LT4 replacement dose in the shortest time after thyroidectomy is important but also challenging. Indeed, in the literature this time is highly variable, ranging from 2 to 120 weeks, with a median of 3.6 months, and only about 30 % reach the euthyroid state at the first postsurgery follow-up [10]. This is not surprising since it is known that BW is not the only factor which predicts the optimal dose of LT4. In fact, several authors suggested the correlation between LT4 requirement and other factors, such as body mass index (BMI) or body surface, gender and menopause, age, and disease causing hypothyroidism. The role of these variables could be explained by the differences in lean body mass (LBM) [11] considering that most metabolic processes, including T4 and T3 deiodination, occur within this body compartment. Moreover, several authors noted that LBM correlates with thyroid and liver size, another important site of T4 conversion. Weaker evidence supports the independent role of gender, age, and BMI [12–14] that also seem to be more likely related to the differences in LBM. However, from a practical point of view, an accurate calculation of LBM requires the use of formulas or techniques that are not cost-effective in the clinical setting, such as, the dual energy X-ray absorptiometry (DEXA). Based on these findings, in the literature few alternative methods are described to calculate the LT4 starting dose after thyroidectomy (Table 23.2) [15–17]. However, no formula has been shown to have a better predictive value than the classic method based only on the patient’s body weight.
Table 23.2
Principal strategies proposed in the literature for the prediction of LT4 requirement after total thyroidectomy for benign disease
Author | Algorithm or formula | |
|---|---|---|
Olubowale et al. [15] Chesterfield, UK | Weight (kg) <53 54–86 87–108 >108 | LT4 dose (μg/day) 100 125 150 175 |
Mistry et al. [16] Hull, UK | LT4 dose (μg/day) = (0.943 × Weight) + (−1.165 × Age) +125.8 (simplified) LT4 dose (μg/day) = Weight – Age + 125 | |
Ojomo et al. [17] Madison, USA | LT4 dose (μg/kg/day) = (− 0.018 × BMI + 2.13) × Weight | |
In a recent study (Di Donna et al.) we readdressed the major predicting factors for the LT4 requirement in order to elaborate a new strategy which could increase the accuracy of the LT4 starting dose after total thyroidectomy. To validate our strategy, we compared it with the different methods currently proposed in the literature. In the first part of the study, a retrospective review of 92 patients (retrospective cohort) who underwent total thyroidectomy for benign disease and started LT4 therapy with a starting dose of about 1.6 μg/kg per day approximated to the nearest marketed formulation was performed; the results obtained by this retrospective analysis were used to formulate a nomogram for the calculation of the LT4 dosage. In the second part of the study, this nomogram was prospectively applied in 31 consecutive patients after total thyroidectomy (prospective cohort). In particular, the patients in the prospective cohort began treatment with LT4 with a recommended dose calculated by the nomogram and approximating to the nearest marketed formulation. The performance of this new strategy has been subsequently confirmed also in larger clinical records (Corsello S.M., personal data, 2015).
Exclusion criteria for both cohorts were: malignancy at histological examination, presence of symptoms or signs of malabsorption, assumption of drugs interfering with LT4 absorption within 4 h following the ingestion of LT4, suspected poor patient’s compliance or eating or drinking anything, except water within 30 min following the assumption of LT4, switch of formulation or brand of LT4. Inclusion criteria for both cohort were: euthyroid state before thyroidectomy, start of LT4 therapy within three days after thyroidectomy, use of the solid formulation and same brand of LT4, check of serum TSH level at 6–8 weeks after thyroidectomy and subsequent checks until the achievement of the euthyroidism, showed by a serum TSH within the range of 0.4–2.5 μUI/mL.
At the first follow-up after 51 ± 16 (mean ± SD) days, only 37 patients in the retrospective cohort were euthyroid (40 %), while 55 required a dose adjustment, 31 because overdosed (34 %) and 24 because underdosed (26 %). Univariate analysis indicated that the strongest correlation with optimal LT4 starting dose was with BW. The other correlations were with BMI, height, age, and preoperative FT3. Based on these observations from the retrospective cohort, an analysis was performed to determine the optimal daily weight-related LT4 dose (expressed as μg/kg/day) in different subgroups based on BMI and age. A univariate analysis revealed that the LT4 dose requirement was inversely related to age and BMI. The comparison between the mean values of the optimal LT4 dose in μg/kg/day in the different age and BMI subgroups revealed that this relationship was statistically significant for both age and BMI. Finally, the LT4 dose in μg/kg was calculated in the 9 subgroups obtained by the combination of previous age and BMI groups. Optimal dose ranged from 1.8 μg/kg/day in patients with lower BMI and younger age to 1.4 μg/kg/day in patients with higher BMI and older age.
Based on these findings a user-friendly nomogram was developed (Fig. 23.2) and then used in the prospective cohort, in which it allowed at the first post-thyroidectomy follow-up (mean ± SD: 50 ± 19 days) to reach the euthyroidism in 21 (68 %) patients, while 6 (20 %) were underdosed and 4 (12 %) overdosed. Moreover, at the first post-thyroidectomy follow-up, overtreatment occurred in the group of elderly patients (age >65 years) in the retrospective cohort in 37 % and in the prospective cohort in no elderly patients.
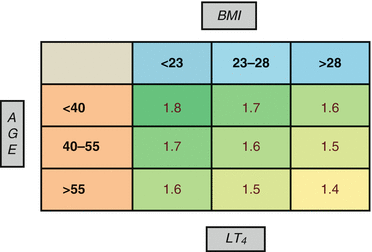

Fig. 23.2
Nomogram for the prediction of LT4 pro kilogram starting dose after total thyroidectomy (Di Donna et al.). BMI kg/m2, age years, LT 4 dose μg/kg/day
An analysis of how the different strategies proposed in the literature would have performed in the retrospective cohort showed that most effective of them would have predicted the correct LT4 starting dose only in 38 of our patients (41 %), and would have been over-replacing in 40 (43.5 %) and under-replacing in 14 (15.5 %).
These data could be explained by the reduction in LBM observed with aging, in addition to the increased prevalence of obesity in elderly patients. Indeed the correlation with age remains significant also in the subgroups analysis by BMI. Perhaps this correlation could be also explained by a possible reduction of thyroxin metabolism in the older age. In regard to gender no statistical difference between women and men was found, while a correlation between height, but not body surface area, and the LT4 optimal dose was reported. The interesting direct correlation between the optimal LT4 dose and the presurgical level of FT3 could be explained by the correlation between the level of the T3 thyroidal production and the rate of conversion of T4 to T3 due to type 1 deiodinase. Also a different sensitivity of thyroid hormones receptor could be involved. More studies to investigate this matter are required.
Serum TSH (and possibly FT4) should be tested in about 6 weeks after starting the replacement therapy. The TSH should be kept in a normal range for patients with benign disease, while a suppressive therapy should be considered in patients with thyroid cancer depending on prognostic risk. An annual TSH or less is sufficient long-term if patients are well clinically. Since its long half-life (about 1 week), LT4 can be started within a week after surgery and it is essential to consider that the steady state of TSH and FT4 is reached after at least 6 weeks and this is the minimum interval after which to evaluate the effect of changes in dosage. The dosage of thyroid hormones and TSH must be performed at fasting in the morning and before to assume LT4, because it can increase FT4 levels also of 20 % about 3 h after assumption and they can remain higher for about 9 h [18]. In special populations, such as elderly patients, pregnant women, patients with heart disease, assuming dugs interfering with LT4 kinetics, undergoing major surgical interventions, poorly compliant, affected by conditions reducing LT4 absorption, and patients with thyroid cancer, a targeted therapy performed by an expert endocrinologist is essential in order to avoid significant oscillations of serum levels of thyroid hormones able to worsen the patient’s outcome.
Finally, it is essential to assume correctly the LT4 therapy, preferably in the morning, at least half an hour before coffee and breakfast, particularly if rich in soy or fiber, and 4–6 h after possible interfering drugs. Also assumption in the evening before sleep and 2 h after last meal is possible, but this approach is less used in clinical practice [19].
23.3 Medical Treatment of Postsurgical Hypoparathyroidism
Hypocalcemia represents a common problem after parathyroidectomy and may be present after thyroidectomy. The reduction of serum calcium is due to hypoparathyroidism, with consequent reduction in bone reabsorption, increased calcium inflow into bone, increased calcium excretion, and decreased intestinal calcium absorption caused by reduced parathyroid hormone (PTH)-mediated renal 1,25-dihydroxycholecalciferol [1,25(OH)2D] synthesis [20]. Hypoparathyroidism represents the most common complication of thyroid surgery, occurring transiently or permanently in 8.3 and 1.7 % of cases respectively [21].
Permanent hypoparathyroidism, defined as continuing need of replacement therapy 6 months after surgery, is an important tool for endocrinologist and it is more common when the goiter is extensive and anatomic landmarks are displaced and obscured. Standard treatment, based on oral calcium and vitamin D supplementation, should normalize serum calcium avoiding side effects such as nephrocalcinosis and nephrolithiasis.
Many authors have studied how to predict postsurgical hypocalcemia. Traditionally, the prediction of hypocalcemia is based on the evaluation after thyroidectomy of serum calcium trends every 6–12 h for 24–48 h, after which levels generally plateau [22]; serum calcium values that suggest treating postsurgical hypocalcemia are under 8 mg/dl [23]. For this reason, patients have to be observed for this period before they can be discharged in order to prevent development of hypocalcemia at home. Postoperative serum calcium less than 7.6 mg/dl on day 1 after thyroidectomy has 95 % specificity in predicting hypocalcemia [24]. Several studies have demonstrated that also PTH assay, performed after completing surgery, is an excellent predictor for the risk of hypocalcemia [25] and multiple algorithms have been offered. In the past, intraoperative PTH monitoring at 5, 10, and 20 min after the excision was proposed as useful to identify patients who will require postoperative calcium supplementation following thyroidectomy [26]. Other authors proposed that a PTH levels reduction of 65 % 6 h after thyroidectomy compared with preoperative values, shows sensitivity and specificity of 96.4 and 91.4 % respectively to predict postoperative hypocalcemia [27]. Specificity and sensibility of 100 % can be reached considering a decrease in PTH of 60 %, coupled with a simultaneous decrease in calcium of 10 %, 5–6 h postoperatively [28]. An unmeasurable PTH one day after surgery (<6.6 pg/ml) predicts a high risk to develop permanent hypoparathyroidism [29]. Recently, also the use of quick-intraoperative intact PTH (QiPTH) performed 15 min after total thyroidectomy (QiPTH 15) has been considered. QiPTH 15 > 15 pg/ml should be able to exclude a postoperative hypoparathyroidism, even if about 30 % of these patients develop transient and mild hypocalcemia within the first postoperative 18 h [30].
The development of hypocalcemia after thyroid surgery can be dependent on several factors. Hyperthyroidism has been shown to have significant effect on bone turn over even after restoring euthyroidism [31]. In fact, patients with Graves’ disease have a dysregulation in calcium homeostasis, because they present lower postoperative serum calcium compared with patients with multinodular goiter, lower calcium/PTH set point, and a significantly increased release of PTH to hypocalcemic stimulus compared with controls [32]. In the first 24 h after total thyroidectomy for hyperthyroidism, transient biochemical hypocalcemia is common, occurring in 60 to 90% of patients [33–36]. Longer period of Graves’ disease and low vitamin D levels represent additional factors contributing to hypocalcemia [37]. The role of vitamin D concentration in affecting calcium kinetics after thyroidectomy is still debated. In fact, it has been shown that low vitamin D levels are associated with 28-fold increase in chances of post total thyroidectomy clinically significant hypocalcemia [38]. On the other hand, some authors exclude that preoperative 25-hydroxycholecalciferol status significantly affects the overall perioperative calcium kinetics [39]. Elderly patients have significant risk factor for postoperative hypocalcemia [38], probably because of decreased intestinal calcium absorption, decreased renal 1α hydroxylation, and decreased cutaneous synthesis of vitamin D. Finally, transient hypoparathyroidism occurs in up to 20 % of patients after surgery for thyroid cancer [40].
Hypocalcemia, evaluated after correction for albumin values, may be associated with a wide field of clinical manifestation, ranging from no or few symptoms (cramps, paresthesia) if hypocalcemia is mild or chronic, to severe life-threatening conditions in case of severe or acute hypocalcemia. In particular, the severity of related symptoms (paresthesias, spasm, tetany, seizures) and signs (Chvostek’s or Trousseau’s signs, impaired cardiac contractility, prolonged QT interval) depends upon both the absolute level of calcium and the rate of decrease. Postsurgical hypocalcemia developing within few hours or few days after surgery (“acute” hypocalcemia), represents a condition in which serum calcium abruptly decreases starting from a condition of normocalcemia or hypercalcemia (primary hyperparathyroidism). For this reason, patients with acute hypocalcemia will be symptomatic at serum calcium values that would not cause symptoms in patients with chronic hypocalcemia.
In patients with acute and symptomatic hypocalcemia, intravenous calcium gluconate therapy will be required, while chronic or mild hypocalcemia can be treated with oral vitamin D and/or oral calcium. Calcitriol (1,25-dihydroxyvitamin D), which is the most active metabolite of vitamin D, is the most used in hypoparathyroidism because PTH should be requested for the renal conversion of calcidiol (25-hydroxyvitamin D) to the active metabolite calcitriol. It has a rapid onset of action (hours), and a biologic half-life of about 4–6 h. However, only few studies evaluated the optimal therapy of hypocalcemia and recommendations are often based on clinical experience.
Neuromuscular irritability with hypocalcemia requires prompt management in hospital and treatment with intravenous calcium. In particular, for symptomatic patients presenting with carpopedal spasm, tetany, seizures, ECG alterations and for asymptomatic patients with an acute decrease in serum corrected calcium to ≤7.5 mg/dL, intravenous calcium therapy is recommended [41]. The preferred form of intravenous calcium is calcium gluconate, because calcium chloride can cause local irritation [42]. One or two 10 ml vials of 10 % calcium gluconate (equivalent to 90–180 mg elemental calcium) should be diluted in 50–100 ml of 5 % dextrose and infused slowly over 10 min [43]. Electrocardiographic monitoring is recommended because dysrhythmias can occur in case of too rapid modification of serum calcium values. This treatment can be repeated until symptoms have cleared and often continuous administration of calcium may be needed to prevent recurrence of hypocalcemia. Patients should also receive oral calcium supplements and calcitriol [42]. The goal of the therapy is to maintain serum calcium at the lower end of the reference range (8 mg/dl). This can be obtained by administering ten 10 ml vials of 10 % calcium gluconate (equivalent to 900 mg of elemental calcium) in 1 l of 5 % dextrose or 0.9 % saline at an initial infusion rate of 50 ml/h. It is important to consider that calcium must be diluted in dextrose or saline to avoid vein irritation and that intravenous solution should not contain bicarbonate or phosphate, because they can form insoluble calcium salts [42]. Intravenous calcium should be continued until the patient is receiving an effective regimen of oral calcium and vitamin D. Moreover, concurrent hypomagnesemia can worse hypocalcemia, by inducing a resistance to PTH action and reducing its secretion. For this reason, if hypomagnesemia occurs, magnesium repletion must be considered. Persistent hypomagnesemia requires oral supplementation (300–400 mg day).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree