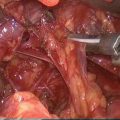
Fig. 7.1
Sequential US images of the moving shot technique. (a, b) The electrode is initially positioned at the periphery of the deep and remote portions of the target nodule, and continuously moves backward (arrows) within the thyroid nodule. (c, d) Next, the electrode tip is relocated in the untreated superficial area, and the ablation is repeated until it covers the entire nodule. (e, f) The procedure is terminated when all of the conceptual units of the targeted nodule have become transient hyperechoic zones
Baek et al. [7] proposed a moving shot technique for thyroid RFA [5–8, 12] (Fig. 7.1). In the fixed-electrode technique, which has been used to treat liver tumors [13–16], the electrode is fixed in position during ablation, resulting in a round ablation zone. A round ablation zone could be dangerous to the surrounding critical structures, because thyroid nodules are ellipsoidal in shape. To resolve this problem, the moving shot technique has been suggested: dividing thyroid nodules into multiple, small conceptual ablation units, and performing RFA unit-by-unit, by moving the electrode tip. The conceptual units are smaller at the margin of the nodules and larger in the central area of the nodules. Initially, the electrode tip is positioned in the deepest, most posterior portion of the nodule to enable the tip to be easily monitored in the absence of any disturbance caused by the transient hyperechoic zone. Ablation is commenced with 20 W (0.5 cm active tip), 30 W (0.7 cm active tip), and 50 W (1.0 cm active tip) of RF power. When a transient hyperechoic zone appears at the targeted unit, RF power starts to decrease, and the electrode tip is moved to an untreated area. The electrode moves continuously both backward and in the superficial direction within the thyroid nodule. The extent of the ablated area is determined by the echogenic area around the electrode. If a transient hyperechoic zone does not appear at the electrode tip within 5 s, RF power is increased in 10-W increments to a maximum of 100 W. If a patient cannot tolerate the pain, injection of a local anesthetic around the thyroid capsule is effective to reduce pain. To treat cystic or predominantly cystic nodules, the cystic fluid is first aspirated, and then RFA is performed. RFA is terminated when all of the conceptual units of the targeted nodule become transient hyperechoic zones (Fig. 7.1). However, certain regions of each thyroid nodule, including those close to critical structures such as the recurrent laryngeal nerve and esophagus, may remain undertreated. Operators must monitor any complications that might occur during and immediately after a procedure. The patient is observed for 1–2 h in the hospital with mild compression of the neck [5, 10].
7.3 Indications
According to the 2012 consensus statement and recommendations of the Korean Society of Thyroid Radiology, RFA can be used to treat both benign thyroid nodules and inoperable, recurrent thyroid cancers in the neck [5–12, 17–24]. The Korean Society of Thyroid Radiology does not recommend RFA for follicular neoplasms or primary thyroid cancers because there is no evidence of a benefit by RFA in these tumors [5, 25]. Caution should be taken regarding the use of thyroid RFA in pregnant women, patients with serious heart problems, and those with contralateral vocal cord palsy [26–30]. Patients with cystic thyroid nodules that regrow after simple aspiration should be treated first with ethanol ablation (EA) rather than RFA [6, 18, 31–33]. A retrospective study and a randomized clinical trial both suggested EA to be a first-line treatment for cystic thyroid nodules [18, 34].
Indications for RFA of benign thyroid nodules include patients with nodule-related clinical problems [5, 7, 8, 10, 11, 22]. The symptom score can be self-measured by patients using a 10-cm visual analog scale (grade 0–10) [6, 7, 31], and the cosmetic score [6, 7, 9, 10, 31] can be measured by a physician (score 1–4: 1, no palpable mass; 2, no cosmetic problem but a palpable mass; 3, a cosmetic problem on swallowing only; and 4, a readily detected cosmetic problem) [6, 7, 18]. Patients with autonomously functioning thyroid nodules (AFTNs) causing thyrotoxicosis are also indicated for RFA [8–10, 12, 35–39]. The nodules with a maximum diameter >2 cm that continue to grow may be considered for RFA.
Indications for RFA of recurrent thyroid cancers include patients at high surgical risk and patients who refuse to undergo repeated surgery [20, 21, 23, 24]. The recommendations suggest that tumor recurrence should be confirmed before treatment by US-guided fine-needle aspiration cytology and/or measurement of the washout thyroglobulin concentration [2, 40]. The treatment strategy of RFA for recurrent thyroid cancers is not well established; however, two strategies have been suggested: complete ablation of any recurrent thyroid cancers visible on US [23, 41] or conservative treatment to improve any symptomatic problems [21]. To achieve complete ablation, several studies have suggested that RFA should be restricted to patients with three or less recurrent tumors in the neck and no metastatic tumors beyond the neck at the time of treatment [2, 23, 41, 42]. Conservative treatment has been applied to treat large recurrent cancers that cause symptomatic problems, such as pain, dysphagia, hoarseness, and dyspnea [21].
7.4 Clinical Outcomes
The efficacy of RFA for benign thyroid nodules can be evaluated by the symptom score, cosmetic score, volume reduction ratio [5, 7, 8], and therapeutic success rate (volume reduction >50 %) [18]. US is recommended for routine follow-up at 1, 6, and 12 months, and every 6–12 months, and changes in size, echogenicity, and intranodular vascularity are evaluated by US. The reduction in volume is calculated using the following equation: volume reduction (%) = ([initial volume (mL) − final volume (mL)] × 100)/initial volume (mL) [5]. The echogenicity of a nodule would be lower than noted before ablation (Fig. 7.2), and intra-nodular vascularity should disappear in regions receiving complete ablation [5]. Additional ablation should be performed if a viable portion of the nodule (i.e., the region showing the same echogenicity as the initial nodule with intra-nodular vascularity) remains on follow-up US, or if a patient complains of incompletely resolved clinical problems, including cosmetic and symptomatic concerns [5, 9]. Proper additional treatment interval is 1–3 months after initial treatment, because rapid volume reduction can be achieved the first 3 months after ablation. Although no guidelines are available to identify a time when follow-up US should cease, additional routine US is not considered necessary when a treated nodule disappears completely or remains as a small scar-like lesion (Fig. 7.2). For the evaluation of AFTNs, a thyroid scan and the measurement of serum thyroid hormone and thyrotropin levels are recommended [8–10]. The efficacy of RFA for recurrent thyroid cancers can be evaluated by the volume reduction ratio, serum thyroglobulin concentrations, and improvement of symptoms [20, 21, 23, 24].
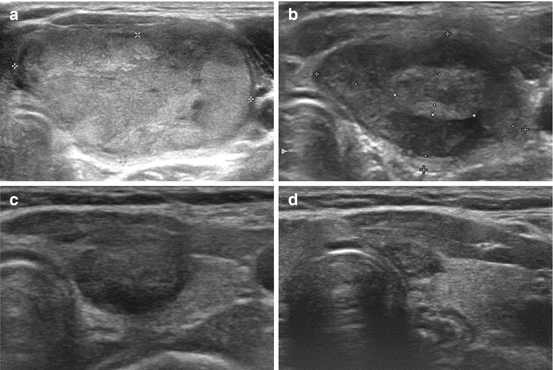

Fig. 7.2
Transverse US images of a well-ablated thyroid nodule. Images obtained before (a), and 12 and 36 months after (b, c) RFA showing the reduced size and decreased echogenicity of the ablated nodule. Only a small scar-like lesion remained at the last follow-up of 56 months (d)
RFA cannot remove thyroid nodules immediately; rather, it induces the necrosis and involution of thyroid nodules, resulting in volume reduction and improvement in clinical symptoms, with an efficacy similar to that of thyroid surgery [5, 10, 11]. Hyperthyroidism caused by AFTNs can be cured or improved by RFA [8–10]. Regarding benign, non-functioning thyroid nodules, the volume reduction after RFA has been found to range from 33 to 58 % at 1 month and from 51 to 92 % at 6 months [5–11, 43]. A long-term study reported that the volume reduction was 90 % at 1 year and 93 % at 4 years [44]. Prospective randomized studies have revealed that RFA is effective for solid thyroid nodules, with 76.5–79.7 % volume reduction at 6 months after RFA [7, 45]. Regarding benign AFTNs, one study [8] reported a volume reduction of 44–81.9 % at 6 months; normalization of thyroid function was 31–87 %. In that study, the volume reduction of treated nodules was slightly lower than that of cold thyroid nodules, possibly due to the autonomy of AFTNs. In addition, incomplete ablation of the nodule margins allowed marginal regrowth of treated nodules, particularly for patients with AFTNs.
Although the moving shot technique can successfully prevent marginal regrowth in many patients, undertreated portions adjacent to the critical structures, as well as large-sized nodules, remain vulnerable to marginal regrowth following RFA [1, 8, 9]. For example, a patient with a large thyroid nodule greater than 20 mL may require additional RFA due to incomplete treatment and unresolved clinical problems [19]. Recent review articles have suggested that RFA is a safe and effective treatment for symptomatic benign thyroid nodules [46–48]. These articles emphasized the proper selection of patients with benign nodules and subsequent monitoring.
7.5 Complications
The reported complications are summarized in Table 7.1. Physicians who perform thyroid RFA should understand the broad spectrum of complications and proper prevention methods [3, 49]. In 2012, the Korean Society of Thyroid Radiology reported various complications for benign nonfunctioning thyroid nodules in a large population multicenter study [49]. The overall complication rate was 3.3 % (48/1459) and the major complication rate was 1.4 % (20/1459). The reported sequelae rate was 0.14 % (2/1459): hypothyroidism in one patient and abscess formation with nodule rupture in the other patient [49]. Regarding the complications related to RFA of recurrent thyroid cancers (Table 7.2), voice change due to recurrent laryngeal nerve injury, skin burn, and procedure-related pain have been reported. Although other nerve injuries have not been reported, any type of injury is possible according to the site of recurrent tumors. Therefore, the operator should monitor the relationship between the nerve and tumor during RFA.
Table 7.1
Complications of RFA for benign thyroid nodules
Kim et al. | Jeong et al. | Deandrea et al. | Spiezia et al. | Baek et al. | Baek et al. | Lee et al. | Sung et al. | Huh et al. | Jang et al. | Faggiano et al. | Ha et al. | Sung et al. | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Year | 2006 | 2008 | 2008 | 2009 | 2009 | 2010 | 2010 | 2011 | 2012 | 2012 | 2012 | 2013 | 2013 | |
No. of patients | 30 | 236 | 31 | 94 | 9 | 15 | 27 | 21 | 15 | 20 | 20 | 11 | 21 | 550 |
Hematoma | 1 | 5 | – | – | – | – | 1 | – | – | – | – | – | – | 7 |
Skin burn | 1 | – | – | – | – | – | – | – | – | – | – | – | – | 1 |
Pain | 1 | 13 | Few | 13 | Few | Few | Few | Few | Few | Few | Few | Few | Few | 27+ |
Transient hyperthyroidism | 3 | 3 | – | – | – | – | – | – | – | – | – | – | – | 6 |
Hypothyroidism | – | – | – | – | 1 | – | – | – | – | – | – | – | – | 1 |
Edema | – | – | 3 | – | – | – | – | – | – | – | – | – | – | 3 |
Fever | – | – | – | 5 | – | – | – | – | – | – | – | – | – | 5 |
Voice change | 1 | 3 | – | – | – | – | – | – | – | –
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|