Peptide receptor radionuclide therapy (PRRT) is a promising new treatment modality for inoperable or metastasized gastroenteropancreatic neuroendocrine tumors patients. Most studies report objective response rates in 15% to 35% of patients. Progression-free (PFS) and overall survival (OS) compare favorably with that for somatostatin analogues, chemotherapy, or newer, “targeted” therapies. Prospective, randomized data regarding the potential PFS and OS benefit of PRRT compared with standard therapies is anticipated.
Key points
- •
Peptide receptor radionuclide therapy (PRRT) is a promising new treatment modality for inoperable or metastasized gastroenteropancreatic neuroendocrine tumors patients.
- •
Most studies report objective response rates in 15% to 35% of patients.
- •
Progression-free and overall survival compare favorably with that for somatostatin analogues, chemotherapy, or newer, “targeted” therapies.
Introduction
In patients with inoperable metastasized gastroenteropancreatic neuroendocrine tumors (GEPNETs), therapeutic options are limited. Treatment with somatostatin analogues decreases hormonal overproduction and can relieve symptoms in patients with GEPNETs. Furthermore, more recent studies showed that treatment with somatostatin analogues prolongs progression-free survival (PFS) in patients with well-differentiated (grades 1 and 2) GEPNETs.
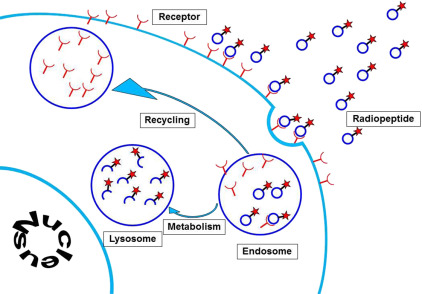
The majority of GEPNETs express somatostatin receptors, mainly somatostatin receptor subtypes 2 and 5. These can be visualized using radiolabeled somatostatin analogues. The first commercially available diagnostic somatostatin analogue was [ 111 Indium-DTPA 0 ]octreotide (Octreoscan; Mallinckrodt, St Louis, MO). Nowadays, newer PET radiopharmaceuticals are available, such as [ 68 Ga-DOTA-Tyr 3 ]octreotide and [ 68 Ga-DOTA-Tyr 3 ]octreotate. A logical sequel to somatostatin receptor imaging for diagnostic purposes was to use the same receptor-binding concept for treatment ( Fig. 1 ).

Introduction
In patients with inoperable metastasized gastroenteropancreatic neuroendocrine tumors (GEPNETs), therapeutic options are limited. Treatment with somatostatin analogues decreases hormonal overproduction and can relieve symptoms in patients with GEPNETs. Furthermore, more recent studies showed that treatment with somatostatin analogues prolongs progression-free survival (PFS) in patients with well-differentiated (grades 1 and 2) GEPNETs.
The majority of GEPNETs express somatostatin receptors, mainly somatostatin receptor subtypes 2 and 5. These can be visualized using radiolabeled somatostatin analogues. The first commercially available diagnostic somatostatin analogue was [ 111 Indium-DTPA 0 ]octreotide (Octreoscan; Mallinckrodt, St Louis, MO). Nowadays, newer PET radiopharmaceuticals are available, such as [ 68 Ga-DOTA-Tyr 3 ]octreotide and [ 68 Ga-DOTA-Tyr 3 ]octreotate. A logical sequel to somatostatin receptor imaging for diagnostic purposes was to use the same receptor-binding concept for treatment ( Fig. 1 ).
Peptide receptor radionuclide therapy efficacy: objective response and survival
Because at that time somatostatin analogues labeled with beta-emitting radionuclides were not available for clinical use, early studies in the 1990s used high activities of the Auger electron-emitting [ 111 In-DTPA 0 ]octreotide for peptide receptor radionuclide therapy (PRRT). These treatments often resulted in symptom relief in patients with metastasized GEPNETs, but objective tumor responses were rare ( Table 1 ).
| Center (Reference) | Ligand | Patient Number | Tumor Response | |||||
|---|---|---|---|---|---|---|---|---|
| CR, n (%) | PR, n (%) | MR, n (%) | SD, n (%) | PD, n (%) | CR + PR (%) | |||
| Rotterdam (Valkema et al, 2002) | [ 111 In-DTPA 0 ]octreotide | 26 | 0 | 0 | 5 (19) | 11 (42) | 10 (38) | 0 |
| New Orleans (Anthony et al, 2002) | [ 111 In-DTPA 0 ]octreotide | 26 | 0 | 2 (8) | NA | 21 (81) | 3 (12) | 8 |
| Milan (Bodei et al, 2003) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide | 21 | 0 | 6 (29) | NA | 11 (52) | 4 (19) | 29 |
| Basel (Waldherr et al, 2001/2002) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide | 74 | 3 (4) | 15 (20) | NA | 48 (65) | 8 (11) | 24 |
| Basel (Waldherr et al, 2002) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide | 33 | 2 (6) | 9 (27) | NA | 19 (57) | 3 (9) | 33 |
| Multicenter (Valkema et al, 2006) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide | 58 | 0 | 5 (9) | 7 (12) | 33 (61) | 10 (19) | 9 |
| Multicenter (Bushnell et al, 2010) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide | 90 | 0 | 4 (4) | NA | 63 (70) | 11 (12) | 4 |
| Copenhagen (Pfeifer et al, 2011) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide | 53 | 2 (4) | 10 (19) | NA | 34 (64) | 7 (13) | 23 |
| Warsaw (Cwikla et al, 2010) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotate | 58 | 0 | 13 (23) | NA | 44 (73) | 3 (5) | 23 |
| Rotterdam (Kwekkeboom et al, 2008) | [ 177 Lu-DOTA 0 ,Tyr 3 ]octreotate | 310 | 5 (2) | 86 (28) | 51 (16) | 107 (35) | 61 (20) | 29 |
| Gothenburg (Sward et al, 2010) | [ 177 Lu-DOTA 0 ,Tyr 3 ]octreotate | 26 | 0 | 6 (38) | NA | 8 (50) | 2 (13) | 38 |
| Lund (Garkavij et al, 2010) | [ 177 Lu-DOTA 0 ,Tyr 3 ]octreotate | 12 | 0 | 2 (17) | 3 (25) | 5 (40) | 2 (17) | 17 |
| Milan (Bodei et al, 2011) | [ 177 Lu-DOTA 0 ,Tyr 3 ]octreotate | 42 | 1 (2) | 12 (29) | 9 (21) | 11 (26) | 9 (21) | 31 |
The next generation of analogues used in PRRT consisted of a modified somatostatin analogue, [Tyr 3 ]octreotide, and a different chelator, DOTA instead of DTPA, which allows stable binding of the β-emitting radionuclide 90 yttrium ( 90 Y). Its maximal tissue penetration is 12 mm and its half-life is 2.7 days. [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide ( 90 Y-DOTATOC) was used in several phase I and phase II PRRT trials in various countries (see Table 1 ). The reported objective responses range from 4% to 33%. Differences in cycle doses and administered cumulative dose, as well as differences in patient characteristics (included tumor types, patient performance status) make it virtually impossible to compare these studies. Different studies report median PFS varying from 17 to 29 months, and median overall survival (OS) from 22 to 37 months ( Table 2 ). In a report on the treatment effects of 90 Y-DOTATOC in a large group of patients, the response to 90 Y-DOTATOC was associated with longer survival.
| Center (Reference) | Ligand | Patient Number | PFS | OS |
|---|---|---|---|---|
| Multicenter (Valkema et al, 2006) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide | 58 | 29 | 37 |
| Multicenter (Bushnell et al, 2010) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide | 90 | 16 | 27 |
| Copenhagen (Pfeifer et al, 2011) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotide | 53 | 29 | — |
| Warsaw (Cwikla et al, 2010) | [ 90 Y-DOTA 0 ,Tyr 3 ]octreotate | 58 | 17 | 22 |
| Rotterdam (Kwekkeboom et al, 2008) | [ 177 Lu-DOTA 0 ,Tyr 3 ]octreotate | 310 | 33 | 46 |
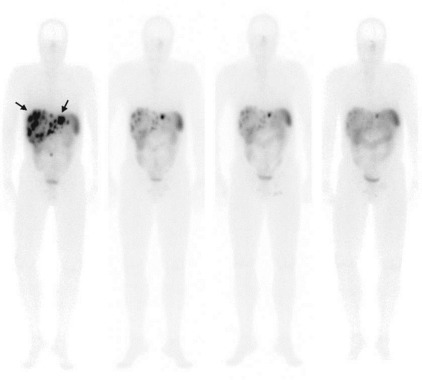
177 Lutetium ( 177 Lu) is a medium energy β-emitter, with a maximal tissue penetration of 2 mm. 177 Lu also emits low-energy γ-rays, allowing scintigraphy after therapy ( Fig. 2 ). The somatostatin analogue [DOTA 0 ,Tyr 3 ]octreotate differs from [DOTA 0 ,Tyr 3 ]octreotide only in that the C-terminal threoninol is replaced with threonine, resulting in a higher affinity for the somatostatin receptor subtype 2 than octreotide. The treatment effects of [ 177 Lu-DOTA 0 ,Tyr 3 ]octreotate ( 177 Lu-octreotate) therapy were described in a large group of GEPNET patients. Complete remission (CR) was found in 5 (2%) patients, partial remission (PR) in 86 (28%), and minor response in 51 (16%; see Table 2 ). Prognostic factors for predicting tumor remission (CR, PR, or minor response) as treatment outcome were high uptake on the Octreoscan, Karnofsky performance score of greater than 70, and low metastatic load to the liver. Median time to progression was 40 months from start of treatment. Progression of disease was more common in patients with extensive disease and in patients who were in a relatively poor general clinical condition (Karnofsky score of <70%, significant weight loss, presence of bone metastases). Several of these factors that had a significant impact on PFS were also found in another study. Median OS was 46 months (see Table 2 ).
Peptide receptor radionuclide therapy: quality of life
In a large group of (nearly 300) patients treated with PRRT and who had a long follow-up, it was shown that quality of life, but also symptomatology improved in 40% to 70% of cases, depending on the preexistence of a certain symptom ( Box 1 ). This is important because the months to years that are gained after PRRT can only be called promising if the time that is gained is free of serious side effects or symptomatology that affect quality of life. By contrast, it was shown that the years gained after PRRT had an improved quality of life, as judged by the patients themselves, using a validated questionnaire.
- •
[ 177 Lu-DOTA 0 ,Tyr 3 ]octreotate: 265 Dutch patients; European Organization for Research and Treatment of Cancer Core Quality of Life-C30 Questionnaire:
- ○
Improved Global Health Score in 36% who scored suboptimal at baseline.
- ○
Improved symptomatology in 40% to 70% of patients who had certain symptoms.
- ○
Peptide receptor radionuclide therapy: acute and subacute side effects
PRRT is generally well-tolerated. Acute side effects are usually mild and self-limiting. Nausea or, more rarely, vomiting is related to the concomitant administration of kidney-protective amino acids. Other, subacute side effects are related to the radiopeptide itself, such as bone marrow suppression, mild hair loss (observed with 177 Lu-octreotate), or, more rarely, an exacerbation of a clinical syndrome ( Box 2 ). The most common subacute side effect of PRRT, occurring within 4 to 6 weeks after therapy is bone marrow suppression. Usually, the hematologic toxicity is mild and reversible. More serious, World Health Organization grade 3 or 4 toxicity may occur, but in less than 15% of patients.