Fig. 19.1
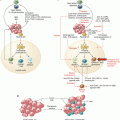
Melanoma cells localized next to CD8+ T cells express PD-L1. Immunohistochemistry staining of a melanoma lymph node lesion for CD8 (brown color), labeling T cells, and PD-L1 (red color), labeling melanoma cells
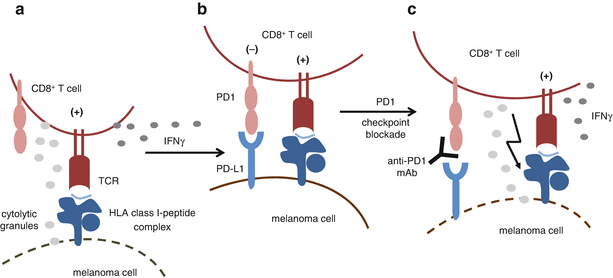
Fig. 19.2
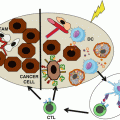
PD1-dependent inhibition of T cell activity by melanoma cells. (a) T cells infiltrating tumor lesions recognize melanoma cells with their T cell receptor (TCR) that binds to specific HLA class I-antigen surface complexes. This leads to the activation of T cells associated with the release of cytolytic granules and the secretion of IFNγ. (b) IFNγ induces expression of PD-L1 on melanoma cells that binds to the inhibitory receptor PD1, thereby dampening T cell activation. (c) Blockade of the interaction between PD1 and its ligand PD-L1 by antibodies releases T cells from inhibitory signaling and restores their activity
Another checkpoint of T cell activation is CTLA-4, the ligands of which are CD80 and CD86 that in contrast to PD-L1 are not expressed on melanoma cells. Instead, both ligands are expressed on professional antigen-presenting cells (pAPC) such as dendritic cells (DC). DCs are equipped to sample tumor antigens in the periphery followed by their migration to draining lymph nodes, where primary T cell activation by tumor antigen-presenting DC takes place [18]. Upon TCR ligation, CTLA-4 is expressed on the surface of T cells that engages CD80/CD86 on pAPC thereby dampening T cell activation [19]. CTLA-4, like PD1, is of importance for the maintenance of self-tolerance. Mice deficient in CTLA-4 show massive lymphoproliferation and early lethality [20]. While expression of CTLA-4 is restricted to T cells, including regulatory T cells (Treg), PD1 is expressed also on natural killer (NK) cells and B cells, and the activity of these cells is most likely affected by antibodies targeting the immune checkpoints. Within this review, we cannot in detail address the complex biology of the receptors and their ligands, but one should keep in mind that PD1 and CTLA-4 can be considered as immune brakes (checkpoints) that limit the effector function of tumor-reactive CD8+ T cells. Release from these brakes (checkpoints) with therapeutic antibodies blocking the receptor-ligand interactions can induce striking and long-lasting clinical responses in melanoma patients as described in the following.
19.4 Checkpoint Blocking Therapy
Before 2011, melanoma patients with advanced metastatic disease received standard palliative treatments with chemotherapeutic agents (dacarbazine, temozolomide, fotemustine). However, large randomized trials demonstrating an impact on overall survival were lacking for these drugs. In 2011, the first checkpoint blocking antibody (ipilimumab, fully human IgG1 antibody) targeting CTLA-4 was approved for therapy of advanced disease patients by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA), based on two randomized trials. One trial compared ipilimumab to a peptide-based vaccine in patients with previously treated unresectable advanced melanoma [21]. In the second trial, patients were treated with ipilimumab plus dacarbazine or dacarbazine plus placebo [22]. Both studies demonstrated a significant improvement for patients being treated with ipilimumab, with response rates between 10 and 15% [21, 22]. Due to the role of CTLA-4 in the maintenance of self-tolerance, its systemic blockade is also associated with toxicity. Severe in some cases, life-threatening adverse events (AE), defined as grade 3–4, occurred in 10–15% of patients receiving ipilimumab monotherapy. The most common grade 3–4 AE were colitis, skin rash, and endocrinopathies [21]. Overall immune checkpoint blockade with ipilimumab was the first therapy with a documented improved overall benefit in a subgroup of melanoma patients. This was a milestone in melanoma immunotherapy (details on CTLA-4-based therapies are given in another chapter of this book) that was quickly followed by success stories based on antibodies targeting the PD1 immune checkpoint.
19.5 Approved Anti-PD1 Antibodies
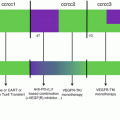
Subsequent to ipilimumab, two antibodies targeting the checkpoint PD1 were approved for the treatment of non-resectable and metastatic melanoma. Approval by the FDA and the EMA was given for nivolumab and pembrolizumab, a fully human and a humanized IgG4 antibody, respectively. Basically, nivolumab- and pembrolizumab-based therapies show much higher durable response rates, and AE occur at a much lower frequency compared to ipilimumab therapy. In the following, we will list the results of some clinical trials relevant to the approval of the antibodies (Table 19.1).
Table 19.1
Phase III trials related to the approval of PD1 checkpoint blocking therapies
Antibody | Trial | Patient cohort | Treatment arms | Outcome | |
|---|---|---|---|---|---|
Monotherapy | Nivolumab | CheckMate 066 (NCT01721772) Robert et al. NEJM 2015 [23] | Unresectable stage III or IV melanoma, no prior systemic treatment, no BRAF mutation (n = 418) | Nivolumab vs. dacarbazine | 1-year OSR: 72.9% vs. 42.1% Median PFS (m): 5.1 vs. 2.2 ORR: 40% vs. 13.9% AE grade 3 and 4: 11.7% vs. 17.6% |
CheckMate 037 (NCT01721746) Weber et al. Lancet Oncol. 2015 [24] | Unresectable stage III or IV melanoma, after ipilimumab or after ipilimumab and BRAFi for BRAF mutants (n = 405) | Nivolumab vs. investigator’s choice of chemotherapy (ICC): dacarbazine or paclitaxel +carboplatin | ORR: 31.7% vs. 10.6% AE grade 3 and 4: 5% vs. 9% | ||
Pembrolizumab | KEYNOTE 066 (NCT01866319) Robert et al. NEJM 2015 [28] | Unresectable stage III or IV melanoma, no more than one prior systemic treatment (n = 834) | Pembrolizumab (every 2 or every 3 weeks) vs. ipilimumab | 6-month PFS: 47.3%, 46.4% vs. 26.5% Estimated 12-month SR: 74.1%, 68.4% vs. 58.2% RR: 33.7%, 32.9% vs. 11.9% AE grade 3–5: 13.3%, 10.1% vs. 19.9% | |
Combination therapy | Nivolumab +ipilimumab | CheckMate 067 (NCT01844505) Larkin et al. NEJM 2015 [29] | Unresectable stage III or IV melanoma, no prior systemic therapies (n = 945) | Nivolumab plus ipilimumab or nivolumab vs. ipilimumab | Median PFS (m): 11.5 vs. 6.9 vs. 2.9 AE grade 3 and 4: 55% vs. 16.3% vs. 27.3% OS not yet available |
19.6 Nivolumab
The FDA approved nivolumab for treatment of advanced melanoma in December 2014, followed by an EMA approval in June 2015 based on the following two studies: CheckMate 066 [23] (randomized, double blind phase III study, 418 melanoma patients included) and CheckMate 037 [24] (open-label phase III study, 405 melanoma patients included) (Table 19.1). In both studies, nivolumab was administered at a dose of 3 mg/kg body weight every 2 weeks.
In the CheckMate 066 trial, patients without prior treatment received either nivolumab or dacarbazine. The 1-year overall survival (OSR) for patients treated with nivolumab was 72.9% compared to 42.1% for dacarbazine. The median progression-free survival (PFS) was 5.1 months for nivolumab and 2.2 months for dacarbazine. The overall response rate (ORR) was 40.0% with nivolumab and 13.9% with dacarbazine, and complete response was observed in 7.6% of the patients treated with nivolumab.
In the CheckMate 037 trial, patients were treated with either nivolumab or chemotherapy (dacarbazine or carboplatin and paclitaxel [investigators choice]). Patients enrolled in this study received pretreatment with nivolumab or BRAF inhibitor (in case of BRAF(V600E) mutant melanoma). The ORR was 31.7% for the nivolumab and 10.6% for the chemotherapy group. Notably, this trial demonstrated that patients pretreated with ipilimumab can still be nivolumab responders. This activity led to accelerated FDA approval of nivolumab in December 2014.
In summary, response rates to nivolumab ranged between 30 and 40%, strikingly higher compared to those reported for ipilimumab treatment. Nivolumab is also much better tolerated. Of course side effects occur, since PD1, like CTLA-4, is also involved in the maintenance of self-tolerance. However, these are less frequent and less severe. In both the CheckMate 066 and the CheckMate 037 studies, the most common side effects in the nivolumab treatment groups were fatigue, pruritus, and nausea. Drug-related AEs of grade 3 or 4 were 11.7% (CheckMate 066) and 5% (CheckMate 037).
19.7 Pembrolizumab
The FDA approved pembrolizumab (formerly known as MK3475 or lambrolizumab) for treatment of advanced melanoma in September 2014, followed by an EMA approval in July 2015 based on the following three trials: the phase I trial KEYNOTE-001 [25, 26], the phase II trial KEYNOTE-002 [27], and the phase III trial KEYNOTE-006 [28].
In the KEYNOTE-001 trial, melanoma patients (n = 135) with or without prior systemic treatment were treated with pembrolizumab at a dose of 10 mg/kg body weight every 2 or 3 weeks or 2 mg/kg body weight every 3 weeks. Across all dose, cohorts of the ORR was 38%, and the overall median PFS was >7 months [25]. In a randomized expansion cohort, melanoma patients (n = 173) showing treatment failure to ipilimumab and BRAF inhibitors (for BRAF(V600) mutant melanoma) were treated with pembrolizumab 2 mg/kg body weight or 10 mg/kg body weight every 3 weeks. There was no significant difference in ORR, median PFS, and 1-year survival rate between both dose cohorts [26]. Notably, this trial demonstrated that patients pretreated with ipilimumab could respond to nivolumab.
In the KEYNOTE-002 randomized trial, patients (n = 540) received pembrolizumab at a dose of 2 or 10 mg/kg body weight or were treated with chemotherapy (investigator’s choice). Patients enrolled in this study were pretreated with ipilimumab and BRAF inhibitors (for BRAF(V600) mutant melanoma). Patients receiving either of the two pembrolizumab doses showed a significantly higher PFS rate after 9 months in comparison to patients treated with chemotherapy: 24 and 29% for pembrolizumab 2 and 10 mg/kg body weight, respectively, compared to 8% in the chemotherapy arm [27].
In the phase III randomized trial, KEYNOTE-006 patients (n = 834) with no more than one prior systemic therapy received either pembrolizumab or ipilimumab (Table 19.1). Pembrolizumab was administered at 10 mg/kg body weight either every 2 or every 3 weeks, whereas ipilimumab was given in four doses of 3 mg/kg body weight every 3 weeks. The 6-month PFS was 47.3 and 46.4% for pembrolizumab administered every 2 and 3 weeks, respectively, compared to 26.5% for ipilimumab. The estimated 1-year survival rate was 74.1% and 68.4% for pembrolizumab given every 2 and 3 weeks, respectively, and 58.2% for ipilimumab. The response rates for pembrolizumab were around 33 and 12% for ipilimumab. Two interim analyses revealed an increase OS for patients treated with pembrolizumab. This led to an early stop of the study, and patients receiving ipilimumab were offered to cross over to treatment with pembrolizumab.
Regarding treatment toxicities, AE were less frequent and less severe in patients treated with pembrolizumab compared to ipilimumab (or chemotherapy). Most common AE were fatigue, diarrhea, endocrine disorders, rash, and pruritus. Drug-related AE of grade 3 or 4 developed in 10–13% of the pembrolizumab-treated patients (administered in different weekly intervals) and in 19.9% of patients receiving ipilimumab.
In summary, response rates to pembrolizumab ranged between 30 and 40%, again strikingly higher compared to those reported for ipilimumab treatment. The activity in pembrolizumab melanoma patients pretreated with ipilimumab led to an accelerated FDA approval. Since testing of different doses and schedules did not reveal significant difference, pembrolizumab received approval in a dose of 2 mg/kg body weight administered every 3 weeks.
19.8 Approved Combined Anti-PD1/Anti-CTLA-4 Combination Therapy
Since PD1 and CTLA-4 receptors bind to different ligands expressed also on distinct cell types, combination therapies were initiated to test the checkpoint blocking antibodies for their synergistic or additive effects. In the CheckMate-067 trial, melanoma patients (n = 945) without prior treatment received a combination of nivolumab and ipilimumab or monotherapy with either ipilimumab or nivolumab (randomization 1:1:1) [29]. The combination therapy mediated a significantly higher PFS (11.5 months) and ORR (57.6%) compared to monotherapy with nivolumab (PFS, 6.9 months; ORR, 43.7%) or ipilimumab (PFS, 2.9 months; ORR, 19.0%). Of the patients treated with the nivolumab/ipilimumab combination, 11.5% developed a complete response compared to 8.9 and 2.2% of the patients receiving nivolumab and ipilimumab monotherapy, respectively. Patients treated with the combination experienced a 58% reduced risk of death or tumor progression compared to ipilimumab monotherapy. The higher response in the combination therapy arm was associated with a higher toxicity: grade 3 and 4 AE occurred in 55% of patients treated with the combination and in 16.3 and 27.3% of patients receiving nivolumab and ipilimumab, respectively. Interestingly, it turned out that in patients with PD-L1-negative tumors (defined by immunohistochemistry), the response rate to the nivolumab/ipilimumab therapy was higher compared to the nivolumab monotherapy, while this difference was not observed for patients with PD-L1-positive tumors. Based on this observation, the FDA approved the nivolumab/ipilimumab combination therapy in 2016 for advanced-stage melanoma patients with PD-L1-negative tumors, while in Europe the combination therapy received approval independently of the tumor’s PD-L1 status.
Overall anti-PD1 and anti-PD1/anti-CTLA-4 therapies induce striking clinical responses in a patient subgroup. But still, a majority of patients is primary resistant to checkpoint blockade, and also acquired resistance is a relevant topic.
19.9 Biomarkers Associated with Response to Anti-PD1 Therapy
To define the patient population that will most likely benefit from anti-PD1 treatment, pretreatment biopsies have been heavily screened for predictive biomarkers that, however, have not been defined yet. Within several clinical trials, expression of PD-L1 in tumor samples has been determined by immunohistochemistry. However, different staining antibodies and different cutoff values were chosen to define PD-L1 positivity rendering results hardly comparable. It seems that response rates to PD1 checkpoint blockade are higher in patients with PD-L1-positive tumors, although patients with PD-L1-negative tumors can respond to anti-PD1 therapy [30, 31]. The presence of infiltrating lymphocytes within the tumor parenchyma but also at the margin of the lesion has been demonstrated to be associated with response to anti-PD1 therapy [31]. This is in line with the concept that CD8+ T cells are reactivated in the course of the treatment and initiate clinical responses. T cells specific for neoantigens might be of highest relevance in this process. According to this, melanoma mutational load determining the amount of neoantigens has been associated with response to anti-CTLA-4 therapy, but so far, this association has not been seen for anti-PD1 treatment [11, 32]. Most likely, it will be necessary to combine multiple biomarkers, some of them to be defined in future studies, in order to predict response to PD1 checkpoint blockade [33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree