Setting
Name
Study design
Primary endpoints
Estimated end of study
First line
CAG209-214
NCT02231749
Nivolumab + ipilimumab vs Sutent
Phase III
PFS and OS
June 2019
First line
IMmotion 151
NCT02420821
Atezolizumab + bevacizumab vs Sutent
Phase III
PFS and OS
June 2020
First line
Javelin Renal 101
NCT02684006
Avelumab + axitinib vs Sutent
Phase III
PFS and OS
June 2018
First line
KEYNOTE-426
NCT02853331
Pembrolizumab + axitinib vs Sutent
Phase III
PFS and OS
December 2019
First line
NCT02811861
Lenvatinib + pembrolizumab vs lenvatinib + everolimus vs Sutent
Phase III
PFS
November 2020
Pembrolizumab, a humanized monoclonal antibody against PD-1, was also investigated in a phase I trial enrolled patients with advanced solid tumors including RCC [43] and is currently being investigated in combination with VEGF-targeted agents.
The combination of pembrolizumab with axitinib is being explored in a phase Ib study of patients with treatment-naive advanced RCC presented at 2016 ESMO Congress [44]. The results reported at ESMO were for 52 patients with advanced RCC who received frontline treatment with axitinib plus pembrolizumab. The ORR was 67.3% (35 patients), including 2 CR and 33 PR. This preliminary analysis indicates that this combination is well tolerated and exhibits antitumor activity in treatment-naïve patients with RCC. An ongoing phase III of this combination versus sunitinib will try to confirm these encouraging results.
Additionally, the combination of pembrolizumab and pazopanib has been investigated in an ongoing phase I/II study. Preliminary data from 20 patients enrolled on the phase I trial were presented. The combination caused significant hepatotoxicity, with 65% of patients (13 out of 20) experiencing grade 3 or higher liver dysfunction (all of whom recovered to grade 1 or lower). The confirmed ORR was 40% (8 out of 20) for the total cohort, 60% for the pazopanib 800 mg group, and 20% of the pazopanib 600 mg group. There was one CR in the pazopanib 800 mg group. New dosing schemas are currently being explored to reduce the risk of hepatotoxicity for the combination of pazopanib with pembrolizumab [45].
Axitinib was further investigated in combination with the anti-PD-L1 avelumab in a phase Ib trial in first-line advanced RCC. Confirmed PR was observed in six patients.
Both pembrolizumab and avelumab were considered encouraging and are being tested in phase III trials in untreated RCC (NCT02853331 and NCT02684006).
Cabozantinib, a dual VEGF/MET inhibitor, was recently demonstrated in a randomized phase III trial after progression on first-line VEGF-targeted therapy to improve ORR and PFS in addition to OS for patients with mRCC [46]. Preclinical evidence showed that MET can promote increased survival of renal cancer cells through the regulation of PD-L1 [47]. The combination of cabozantinib plus nivolumab alone or in combination with ipilimumab in patients with genitourinary tumors is currently accruing patients (NCT02496208).
Lenvatinib is a multitarget TKI of VEGF with activity against FGFR, PDGFR, RET, and KIT. A phase II randomized, open-label study investigated lenvatinib (24 mg/day), everolimus (10 mg/day), or the combination (lenvatinib 18 mg/day and everolimus 5 mg/day) as second-line treatment in mRCC (150 patients) [48].
Safety and efficacy of lenvatinib in combination with pembrolizumab, a humanized monoclonal antibody to PD-1, are currently being explored in a phase Ib/II study (NCT02501096).
Tivozanib is a potent and selective inhibitor of all three VEGF receptors. In a phase I/II trial in combination with nivolumab in RCC, known as the TiNivo trial, the safety of tivozanib in combination with nivolumab at escalating doses of tivozanib is being evaluated, and, assuming favorable results, the trial will be followed by an expansion phase II cohort at the established combination dose (EudraCT2016002310-44).
A summary of the activity of VEGF inhibition and PD-1/PD-L1 blockade in phase I trials is shown in Table 21.2.
Table 21.2
Activity of the combination of VEGF inhibition and PD-1/PD-L1 blockade in RCC phase I trials
Phase I trial | Patients, number | ORR, % | CR, % | PR, % |
|---|---|---|---|---|
Nivolumab + sunitinib [40] | 33 | 52 | Not reported | Not reported |
Nivolumab + pazopanib [40] | 20 | 45 | Not reported | Not reported |
Avelumab + axitinib [52] | 6 | 100 | 0 | 100 |
Pembrolizumab + axitinib [44] | 52 | 71.2 | 5.8 | 65.4 |
Nivolumab + cabozantinib [53] | 23 | 43 | 4 | 39 |
Pembrolizumab + lenvatinib [54] | 13 | 69.2 | 0 | 69.2 |
Conclusion
PD-1 blockade has produced encouraging response rates in RCC. Especially nivolumab has met its primary endpoint of benefit in OS compared to everolimus in the CheckMate 025 trial becoming a new standard of care. Combination of anti-PD-1/PD-L1 and other agents against RCC has demonstrated higher response rates than single agent alone for the price of higher toxicities.
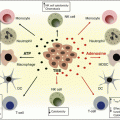
Development of predictive biomarkers to determine which patients will derive clinical benefit from these treatments remains an important need and is an area of active investigation. PD-L1 expression by IHC has been extensively evaluated as a predictive biomarker. Multiple unsolved issues have confounded its use: different antibodies, variable IHC thresholds to determine positivity, adequate tissue preparation and processing, divergent expression between primary and metastatic foci, and evaluation of staining of tumor versus immune cells [49].
In spite of efficacy and toxicity profile of immune checkpoint inhibitors, a series of open questions remains.
First, what is the role of PD-1 blockade in RCC subpopulations, such as patients with non-clear cell RCC? Non-clear cell RCC patients have largely been excluded from the trials mentioned above. Recently, PD-L1 status of patients with non-clear cell RCC was assessed and correlated with OS, suggesting that blockade of PD-1/PD-L1 axis may show beneficial in this population [12].
Second, are traditional measures of assessing response to therapy still predictive of the benefit of checkpoint inhibitors? In the phase III trial of nivolumab, a significant benefit in OS was observed in patients who received nivolumab compared to patients who received everolimus, without a PFS benefit [28]. This modest effect in PFS is probably the result of the pseudo-progression, which could occur due to infiltration of tumors by activated immune cells and could mimic progression. Immune-related Response Evaluation Criteria in Solid Tumors (iRECIST) have been proposed to address this phenomenon [50].
Third, the optimal duration to continue immunotherapy is also unknown. Some trials have stopped therapy at time of progression or demonstrating intolerance. Others had proposed a predefined stopping point of 2 years of therapy. Whereas immunotherapy response patterns differ from traditional therapies, some patients may benefit from nivolumab also after RECIST progression [51].
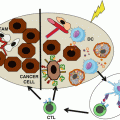
Probably, an improved understanding of the host immune system and tumor microenvironment will better elucidate which patients take advantage from these promising agents. Complementary PD-1 pathway blockade and targeted therapy or other immunologic agents have the potential to reduce the tumor-induced immunosuppression and improve clinical outcomes for patients with mRCC. Given the encouraging clinical activity and safety profile of the current PD-1/PD-L1 inhibitors, it is likely that combination approaches will become key components.
Studies exploring novel agents, combinations, as well as biomarkers of response are currently ongoing and will likely inform the future treatment landscape for patients with mRCC.
References
1.
2.
Crisci A, et al. Spontaneous regression of lung metastases from renal cell carcinoma: the importance of immunogenetic factors and a review of the literature. Minerva Urol E Nefrol Ital J Urol Nephrol. 2008;60:123–35.
3.
Ueda K, et al. Spontaneous regression of multiple pulmonary nodules in a patient with unclassified renal cell carcinoma following laparoscopic partial nephrectomy: a case report. Mol Clin Oncol. 2016;5:49–52.CrossrefPubMedPubMedCentral
4.
Negrier S, et al. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Français d’Immunothérapie. N Engl J Med. 1998;338:1272–8.CrossrefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree