Fig. 7.1
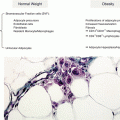
The etiological, hormonal, and obstetric clinical outcomes of PCOS (Modified from Teede et al. [38])
7.3 PCOS: Infertility and Early Pregnancy Loss
The reproductive dysfunction in women with PCOS relates both to anovulation and to early pregnancy loss. Anovulation is the cause of infertility in about one third of couples seeking infertility treatment, and PCOS accounts for 90 % of those cases [1]. To conceive many of PCOS women require assisted reproductive techniques, such as ovulation induction or IVF [12].
Complications associated with PCOS such as hyperandrogenemia, hyperinsulinemia, and obesity have a major impact on ovulation and pregnancy outcome (Fig. 7.1). Miscarriage rate is high in PCOS women and varies between 15 and 35 % of all conceptions. This may be explained by the negative effect of the whole array of cofactors associated with PCOS, metabolic dysfunction, low-grade inflammation, endocrine abnormalities on ovulatory function, oocyte quality, and endometrial receptivity. Ovarian hyperandrogenism and hyperinsulinemia may promote premature granulosa cell luteinization, and paracrine dysregulation of growth factors may disrupt the intrafollicular environment and impair cytoplasmic and nuclear maturation of oocytes ([13] Amsterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group). Endometrial receptivity is disrupted by a number of altered mechanisms. The overexpression of androgen receptors in the endometrium and reduced biomarkers of endometrial receptivity to embryonic implantation contribute to lower implantation rates in women with PCOS. Elevated estrogen, low levels of progesterone, no ovulation, and hyperinsulinemia with elevated free IGFs all contribute to abnormal decidualization and increased miscarriage rate [18].
These features are not universal to all women with PCOS, as oocyte quality, fertilization, and implantation rates can be normal in some women with PCOS [32]. Diverse differences in fertility and pregnancy outcome between women with PCOS are clinically challenging, and risk factors need to be identified in this group of women to improve management of reproduction.
7.4 PCOS and Adverse Pregnancy Outcome
Several studies suggest that PCOS has a negative impact on pregnancy outcomes with an increased risk of gestational diabetes, pregnancy-induced hypertension, preeclampsia, preterm birth PTB, and need of cesarean section, even in the absence of obesity [2, 3, 26, 31]. A greater risk of neonatal complications has been reported and includes higher neonatal intensive care admissions; neonates were shown to have significantly increased risk of meconium aspiration, lower birth weights, and a low Apgar score (<7 at 5 min) [31]. In addition, multiple pregnancy contributes to increased risk of perinatal morbidity observed following fertility treatments of PCOS women [12].
PCOS pregnancies are further complicated by hyperinsulinemia and an increased risk of gestational diabetes. Normal pregnancy induces a state of insulin resistance which may result in impaired glucose tolerance or gestational diabetes mellitus. Since women with PCOS are predisposed to glucose intolerance, they are at increased risk of developing gestational diabetes. In addition obesity affects 5–40 % of pregnant women with PCOS [39] which can also increase the risk of gestational diabetes. Mexican women with a history of infertility and PCOS have 2.8 times greater risk of developing gestational diabetes when compared to Caucasians [30].
The prevalence of gestational hypertension and preeclampsia is estimated at 10–30 and 8–15 %, respectively, in women with PCOS. This prevalence may be even higher when PCOS women are obese and hyperinsulinemic.
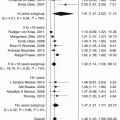
Preterm delivery complicates 6–15 % of pregnancies in women with PCOS [41]. However, PCOS by itself is not an independent risk factor because these patients have more assisted reproductive technologies, more chance of multiple pregnancies, and pregnancy-induced hypertension contributing to more preterm deliveries [17]. A high frequency of cervical insufficiency was found in PCOS, with a prevalence in the range of 3 % and incidence rate of 18 per 1,000 births, particularly in South Asian and Black women [14]. Indeed, the increased risk of preterm birth is confined to hyperandrogenic women with PCOS who had a twofold increased risk of preterm delivery and preeclampsia compared to non-hyperandrogenic ones.
7.5 PCOS and Adverse Pregnancy Outcome: Pathogenetic Mechanisms
The spectrum of pregnancy complications associated with PCOS may be driven by impaired decidual trophoblast invasion and defects in placentation, related to hormonal and metabolic dysfunction. Alterations in the endovascular trophoblast invasion and in the macroscopic and microscopic structure of the placenta have been reported [29].
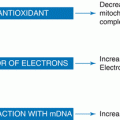
The alterations in early trophoblast invasion and placentation detected in PCOS patients vary among PCOS phenotypes. In particular, a higher rate of abnormalities in relation to trophoblastic invasion and placental development was observed in patients with hyperandrogenism and ovarian dysfunction regardless of whether they were diagnosed with PCOS or not. PCOS patients who had a complicated pregnancy were reported to have more severe serum androgen concentrations and insulin sensitivity indexes in comparison to subjects without any pregnancy and/or neonatal complications [11]. However, the exact mechanisms by which hyperandrogenemia and insulin resistance/compensatory hyperinsulinemia affect endovascular trophoblast invasion and induce placental alterations are unknown. Furthermore, the comorbidity of obesity may increase the risk of adverse pregnancy outcome in PCOS through enhanced hyperandrogenism, hyperinsulinemia, and, since excess adipose tissue acts as an endocrine and inflammatory organ, altered concentrations in leptin, TNF-α, and IL-6 [23] (Fig. 7.2). This obese phenotype of PCOS adds to possible abnormal trophoblastic invasion the preexisting endothelial dysfunction that might damage women affected by metabolic syndrome [9].
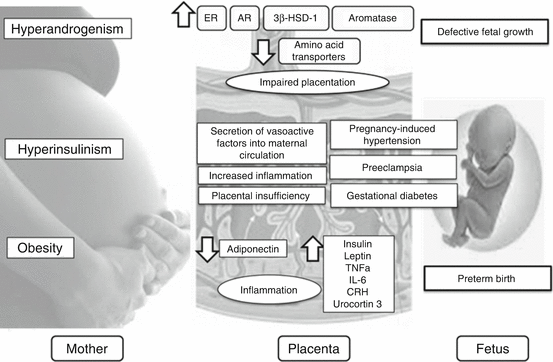

Fig. 7.2
Pathogenetic mechanisms of obstetric complications in PCOS
7.6 Hyperandrogenism
Ovarian hyperandrogenism is a cardinal feature of PCOS. Intrinsic amplified steroidogenetic capacity of theca cells results in increased ovarian androgen secretion. Several endocrine mechanisms may contribute to hyperandrogenism, and these include pituitary LH hypersecretion, relative FSH insufficiency, and high levels of insulin and AMH inhibiting aromatase activity. Obesity also amplifies hyperandrogenism in PCOS resulting in increased total testosterone, free androgen index, and decreased sex hormone-binding globulin (SHBG). Obese women with PCOS exhibit a higher degree of insulin resistance and compensatory hyperinsulinemia which contributes to androgen excess.
A number of studies have reported elevated levels of some, but not all, circulating androgens during normal pregnancy from the first trimester and toward term. In contrast, maternal circulating dehydroepiandrostenedione sulfate (DHEAS) levels fall across gestation to 50 % of the nonpregnant levels, and SHBG levels increase during the first trimester of pregnancy and continue to increase dramatically throughout mid- and late gestation. In the fetus, levels of some androgens are dependent on fetal sex; in fact in fetal blood, testosterone levels are higher in males, whereas dihydrotestosterone levels are similar in both sexes. Although testosterone levels are higher in the fetal compartment of male fetuses, there is no association between fetal sex and maternal serum concentrations of any androgen [23].
Elevated androgen levels during pregnancy are associated with low birth weight in humans and animals, and women experiencing hyperandrogenism associated with PCOS and preeclampsia have a higher than normal prevalence of small for gestational age newborn babies at delivery. Increased maternal testosterone concentrations do not cross the placenta to directly suppress fetal growth but affects amino acid nutrient delivery to the fetus by downregulating specific amino acid transporter activity. Thus, the fetus appears to be protected from excess maternal androgen perhaps due to effective enzymatic inactivation by the placenta, and in the absence of these protective mechanisms, high levels of androgens would cause hirsutism and virilization of both mother and female fetus.
Maternal mechanisms that protect the fetus from androgens include the physiological increase of maternal circulating SHBG and the pregnancy-induced rapid elevation of progesterone and androstenedione resulting in an inhibition of the conversion of testosterone to dihydrotestosterone. The fetus is further protected by the placenta which metabolizes androgens; in particular the placental aromatase complex rapidly converts testosterone or androstenedione to estrone and estradiol, respectively [23].
Fetal growth restriction may arise in the presence of hyperandrogenism by the specific downregulation of amino acid transporters within the placenta which results in a decrease of available nutrients to the fetus [34]. A rat model of excess maternal androgen reported a decrease in placental size, increased circulating androgens and increased circulating estradiol concentrations, and increased expression of androgen and estrogen receptors, potentially affecting the ability of the placenta to deliver nutrients to the fetus [36].
Other testosterone-induced alterations in endometrial, stromal, decidual, and placental function could influence fetal growth and pregnancy outcome in PCOS pregnancies. Testosterone regulates the endometrial expression of homeobox A10 (HOXA10) [5], and it is proposed that hyperandrogenism could impair the cell–cell and cell–extracellular matrix interactions during the first phases of pregnancy, thereby reducing HOXA10 expression. Furthermore, dihydrotestosterone exerts a direct effect on the ultrastructural changes associated with decidual transformation in human endometrial stromal cells. These early pregnancy events induced by androgens could reduce implantation success and increase the risk of miscarriage. Later in gestation testosterone can promote placental macrophage- and trophoblast-mediated low-density lipoprotein oxidation and cytotoxicity which could affect fetal development. Finally, the human placenta expresses CYP17 and produces androgens de novo, and a higher 3β-hydroxysteroid dehydrogenase type 1 activity and a lower P450 aromatase activity were detected in the placental tissue of PCOS patients, suggesting that the steroidogenic function of the placenta in PCOS women is altered per se, contributing to a higher androgen concentration observed in maternal blood of PCOS patients and therefore increasing the effects of hyperandrogenism in pregnancy [24].
7.7 Hyperinsulinism
Hyperinsulinemia can have multiple effects on fertility, pregnancy success, and fetal growth and well-being. PCOS-related secondary hyperinsulinemia may interfere with paracrine, and perhaps autocrine, signaling in the endometrium, causing inadequate cell differentiation during the first phases of pregnancy. In addition, the reduction in IGFBP-1 could increase the mitotic effect of insulin-like growth factor (IGF)-I on the endometrium [4], whereas the reduction of glycodelin could decrease the physiological immune suppression observed during the early phases of gestation and alter the modulation of trophoblast invasiveness. In the endometria of hyperinsulinemic hyperandrogenic PCOS patients, there is a reduced insulin-related expression of the transmembrane glucose transporter 4 (GLUT4) reducing the glucose uptake, with subsequent alterations in the metabolism of the endometrial cells.
During pregnancy, PCOS women are predisposed to peripheral tissue resistance to insulin, with consequent hyperinsulinism and fetal hyperglycemia. Moreover, hyperandrogenemia and hyperinsulinemia observed during pregnancy in PCOS women result in an abnormal lipid profile and a decrease in adiponectin concentrations, predisposing women to the onset of gestational diabetes [35].
Hyperinsulinemia in PCOS women is also associated with having low levels of insulin-like growth factor binding globulin-1 (IGFBP-1), a regulator of the activity of IGF-1, which may be implicated as a cause of higher incidence of growth abnormalities of the fetus and increased risk of preeclampsia.
7.8 Inflammation and Obesity
Clinical data demonstrated that hyperandrogenic and/or hyperinsulinemic PCOS patients are characterized by an abnormal pattern of low-grade chronic inflammation that may be responsible for abnormal immune regulation during pregnancy in PCOS patients, thereby increasing the frequency and the extent of immune-mediated placental pathologies [33] and possibly increasing the risk of preterm birth in women with PCOS.
PCOS has been characterized by chronic low-grade inflammation, with complex associations to insulin resistance, visceral adiposity, hyperandrogenism, and resultant increased production of specific cytokines and chemokines including TNF-alpha, IL-6 and IL-1, adhesion molecules, follistatin, and C-reactive protein. Furthermore, a panel of six proteomic biomarkers was similarly expressed in women with preterm birth and women with PCOS compared to their respective controls, including pyruvate kinase M1/M2, vimentin, fructose bisphosphonate aldolase A, heat-shock protein beta-1, peroxiredoxin-1, and transferrin, which could be potentially used to better understand the pathophysiological mechanisms linking PCOS and preterm birth [16].
Molecular and biochemical findings support the theory that in some situations, labor may be viewed as part of an inflammatory cascade model, including pathways involving specific leukocyte subsets and proinflammatory mediators. As such, underlying inflammatory mediators associated with PCOS may also contribute to predisposition for preterm delivery. This is an area that requires further investigation.
7.9 Long-Term Effects of PCOS
It is now well established that women with PCOS are at increased risk for developing diabetes mellitus, glucose intolerance, hypertension, hyperlipidemia, the metabolic syndrome, and cardiovascular disease. Although the high prevalence of insulin resistance in women with PCOS, especially those who are also obese, has been largely held accountable for these long-term sequelae, there is now emerging evidence for a role of androgen excess in these processes which is more than merely permissive [20]. There is a strong association between hyperandrogenemia, an increased free androgen index, and the metabolic syndrome in premenopausal women with or without PCOS ([13] Amsterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group). Moreover, among obese hyperandrogenic adolescents, hyperandrogenemia was found to be a significant predictor of the metabolic syndrome, independent of obesity and insulin resistance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree