The indications for peritonectomy + hyperthermic intraperitoneal chemotherapy (HIPEC) are based on careful assessment of disease extent, but no imaging procedure is accurate enough to identify lesions smaller than 5 mm or extensively diffuse. Video-laparoscopy allows, with minimal surgical trauma, correct staging with a reliable prediction of expected cytoreduction index. Operative laparoscopy is indicated for palliation of neoplastic ascites with chemotherapy, offering encouraging results. Minimally invasive surgery in the treatment of minimal peritoneal carcinomatosis is not yet validated from wide international experience; the procedure is technically possible with strict indications, and combination with intraoperative hyperthermic chemotherapy is strongly recommended.
- •
Peritonectomy + hyperthermic intraperitoneal chemotherapy (HIPEC) shows good late results only for patients in whom a complete cytoreductive surgery was performed; the expected completeness of cytoreduction (CC0) is the cornerstone of the indications to the treatment.
- •
Imaging (computed tomography [CT] and CT/positron emission tomography [PET]) is still considered the first-choice diagnostic test in the workup of peritoneal carcinomatosis, but video-laparoscopic (VLS) staging is the only presidium that allows a correct staging and a reliable forecast of the expected CC0.
- •
The VLS staging technique is safe and reliable, not presenting major complications and mortality.
- •
In refractory malignant ascites not suitable for surgery, laparoscopic HIPEC shows encouraging results regarding both the ascites and the improvement in the Karnofsky index.
- •
VLS peritonectomy might be used in minimal carcinomatosis, confined to 1 or 2 sectors and well-defined histologic types. At present, in the absence of comparative data with open surgery, it is not possible to consider it as a gold standard in minimal carcinomatosis.
Introduction
The integrated approach to peritoneal carcinomatosis is based on the change of paradigm introduced by Paul Sugarbaker, who considers this abnormality as a locoregional disease in which only the abdominal compartment is involved. Following this approach, and carrying out an accurate assessment of locoregional tumor burden, patients can be selected for treatment with a procedure that combines surgery (peritonectomy) and hyperthermic intraperitoneal chemotherapy (HIPEC).
The first step of selection consists in excluding from the integrated treatment all the patients with hematogenous metastases in the extra-abdominal districts and with nonresectable liver metastases.
Of paramount importance is the qualitative and quantitative assessment of locoregional tumor burden and the forecast of the extent of resection needed to achieve complete cytoreduction; the main purpose of this assessment is to select for treatment those patients who can achieve a real improvement in survival, also carefully evaluating the operative risk.
Peritonectomy + HIPEC, when a complete cytoreduction is accomplished, permits an overall survival (OS) and a disease-free survival (DFS) that cannot be achieved by any other kind of treatment.
Diagnosis and exclusion of patients with extraregional disease or high peritoneal cancer index: imaging
The first role of imaging is to rule out the presence of distant metastases in extra-abdominal areas, which is an absolute criterion of exclusion. The lesions pertaining to peritoneal carcinomatosis can be demonstrated directly through ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT.
Ultrasonography is useful in detecting ascites, large abdominal masses, or liver metastases. CT gives valid topographic representation of the abdominal cavity and a precise definition of site, type, and extent of the pathologic process.
The main findings for peritoneal carcinomatosis on CT scan are focal or diffuse thickening of peritoneal folds, appearing as sclerotic, jelly-like, reticular, reticulonodular, nodular, or in large plaques. According to such an appearance, it is possible to identify different types of peritoneal carcinomatosis, namely infiltrative, micronodular (miliary), and macronodular (nodous) forms, with some overlapping among them.
Nodular or plaque lesions can show various levels of enhancement after intravenous injection of contrast media, or even a little attenuation, with cyst-like appearance; sometimes it is possible to see calcification deposits inside the lesions.
Mesenteric involvement is often of the sclerotic type, with thickening and retracted appearance of the single layers. In the greater omentum it is often possible to see reticular/micronodular, or a nodular aspect and/or large plaques, in some cases where a huge and thick neoplastic tissue layer of inhomogeneous density is located between the abdominal wall and the bowel loops (“omental cake”).
Chances of identifying nodular lesions depend on the anatomic location; small nodules (<5 mm) are more easily recognized on the surfaces of liver or spleen.
Image visualization on different planes (multiplanar reconstruction sagittal, coronal, oblique) can help in searching for lesions on curved structures such as diaphragm, paracolic gutters, and small-intestine loops.
Small-bowel and large-bowel loops can be warped and attached to each other, capable of causing stenosis and mechanical intestinal obstruction. Ascites is present in more than 70% of cases, free or entrapped; CT scan can demonstrate free ascites if the amount is more than 50 mL. In the upper part of the abdomen the fluids collect first in the hepatorenal space and in the subphrenic right and left spaces; in the lower part the initial collection is in the Douglas pouch, then around urinary bladder and in paracolic gutters.
Primary tumor can also be identified (if it has not yet been surgically removed); if not possible, the synchronous peritoneal lesion can be assumed as primary, or a spread from an extra-abdominal focus (eg, breast cancer, melanoma) might be hypothesized.
In the differential diagnosis, some rare diseases causing similar lesions should be considered: tuberculous peritonitis, mesenteric panniculitis, diffuse peritoneal leiomyomatosis, and extramedullary hematopoiesis.
Pseudomyxoma peritonei can grow close to the original lesions (localized form) or spread to most of the peritoneal surface (diffuse form). CT shows solid, inhomogeneous tissue, mostly hypodense, localized around the peritoneal sides, which usually appears thickened by the irritating action of mucin; ascites is often present. As for peritoneal carcinomatosis, even a pseudomyxoma peritonei fibrotic reaction can cause adhesions with obstruction of the intestinal transit.
CT can help in the evaluation of intra-abdominal metastatic diffusion, and in choosing the best treatment option when involvement of liver or head of pancreas is present and when the mesenteric root is grossly infiltrated; in the preoperative evaluation of the peritoneal cancer index (PCI), it shows 88% sensitivity and 12% accuracy. CT shows low sensitivity in assessing small-bowel lesions (8%–17%), which decreases to 11% for lesions smaller than 5 mm in all quadrants with a significant underestimation of clinical PCI.
When the CT scan is positive, mainly in patients with bulky tumors, its specificity reaches 100% in all regions. CT is very helpful during the early postoperative period because it can identify complications such as fluid collections, abscesses, perforations, fistulae, and pancreatitis.
Magnetic nuclear resonance shows no advantages when compared with CT in the evaluation of PCI and in the prediction of achievable cytoreduction index.
18 F-FDG PET, if used alone, underestimates PC. 18 F-FDG PET/CT shows instead 90% sensitivity and preoperative specificity of 77% for degree II and III lesions; nevertheless it underestimates small lesions in all locations, when the size of the nodules is less than 5 mm. In the follow-up period, CT can detect early recurrences, even if a differential diagnosis between true relapses and fibrotic scars caused by treatments is often difficult; in these patients 18 F-FDG PET/CT can help to make a differential diagnosis.
Usually 18 F-FDG PET/CT is positive after completeness of cytoreduction index grade 2 (CC2) to CC3 and negative after CC0 to CC1.
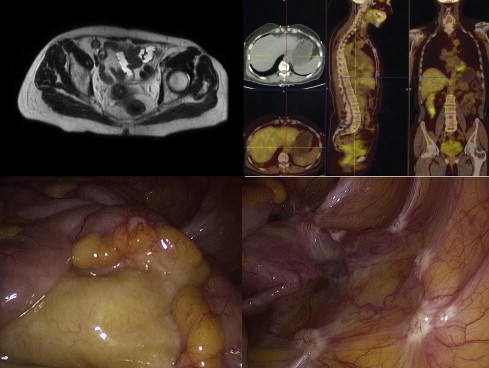
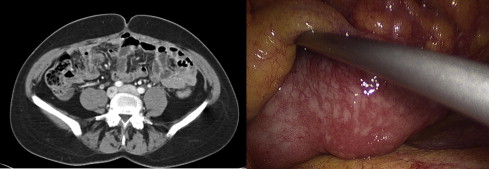
Not all histologic types show good glucose uptake at 18 F-FDG PET/CT; it could therefore be advisable to perform a preoperative examination to assess the initial glucose uptake to exclude false negatives in the follow-up period of nonuptaking tumors. Recent studies showed that both CT and 18 F-FDG PET/CT were unable to give a correct staging of carcinomatosis. One may conclude that nowadays there is no noninvasive procedure that can correctly evaluate PCI and expected cytoreduction index after treatment, especially if the lesions are small ( Figs. 1 and 2 ).
Diagnosis and exclusion of patients with extraregional disease or high peritoneal cancer index: imaging
The first role of imaging is to rule out the presence of distant metastases in extra-abdominal areas, which is an absolute criterion of exclusion. The lesions pertaining to peritoneal carcinomatosis can be demonstrated directly through ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT.
Ultrasonography is useful in detecting ascites, large abdominal masses, or liver metastases. CT gives valid topographic representation of the abdominal cavity and a precise definition of site, type, and extent of the pathologic process.
The main findings for peritoneal carcinomatosis on CT scan are focal or diffuse thickening of peritoneal folds, appearing as sclerotic, jelly-like, reticular, reticulonodular, nodular, or in large plaques. According to such an appearance, it is possible to identify different types of peritoneal carcinomatosis, namely infiltrative, micronodular (miliary), and macronodular (nodous) forms, with some overlapping among them.
Nodular or plaque lesions can show various levels of enhancement after intravenous injection of contrast media, or even a little attenuation, with cyst-like appearance; sometimes it is possible to see calcification deposits inside the lesions.
Mesenteric involvement is often of the sclerotic type, with thickening and retracted appearance of the single layers. In the greater omentum it is often possible to see reticular/micronodular, or a nodular aspect and/or large plaques, in some cases where a huge and thick neoplastic tissue layer of inhomogeneous density is located between the abdominal wall and the bowel loops (“omental cake”).
Chances of identifying nodular lesions depend on the anatomic location; small nodules (<5 mm) are more easily recognized on the surfaces of liver or spleen.
Image visualization on different planes (multiplanar reconstruction sagittal, coronal, oblique) can help in searching for lesions on curved structures such as diaphragm, paracolic gutters, and small-intestine loops.
Small-bowel and large-bowel loops can be warped and attached to each other, capable of causing stenosis and mechanical intestinal obstruction. Ascites is present in more than 70% of cases, free or entrapped; CT scan can demonstrate free ascites if the amount is more than 50 mL. In the upper part of the abdomen the fluids collect first in the hepatorenal space and in the subphrenic right and left spaces; in the lower part the initial collection is in the Douglas pouch, then around urinary bladder and in paracolic gutters.
Primary tumor can also be identified (if it has not yet been surgically removed); if not possible, the synchronous peritoneal lesion can be assumed as primary, or a spread from an extra-abdominal focus (eg, breast cancer, melanoma) might be hypothesized.
In the differential diagnosis, some rare diseases causing similar lesions should be considered: tuberculous peritonitis, mesenteric panniculitis, diffuse peritoneal leiomyomatosis, and extramedullary hematopoiesis.
Pseudomyxoma peritonei can grow close to the original lesions (localized form) or spread to most of the peritoneal surface (diffuse form). CT shows solid, inhomogeneous tissue, mostly hypodense, localized around the peritoneal sides, which usually appears thickened by the irritating action of mucin; ascites is often present. As for peritoneal carcinomatosis, even a pseudomyxoma peritonei fibrotic reaction can cause adhesions with obstruction of the intestinal transit.
CT can help in the evaluation of intra-abdominal metastatic diffusion, and in choosing the best treatment option when involvement of liver or head of pancreas is present and when the mesenteric root is grossly infiltrated; in the preoperative evaluation of the peritoneal cancer index (PCI), it shows 88% sensitivity and 12% accuracy. CT shows low sensitivity in assessing small-bowel lesions (8%–17%), which decreases to 11% for lesions smaller than 5 mm in all quadrants with a significant underestimation of clinical PCI.
When the CT scan is positive, mainly in patients with bulky tumors, its specificity reaches 100% in all regions. CT is very helpful during the early postoperative period because it can identify complications such as fluid collections, abscesses, perforations, fistulae, and pancreatitis.
Magnetic nuclear resonance shows no advantages when compared with CT in the evaluation of PCI and in the prediction of achievable cytoreduction index.
18 F-FDG PET, if used alone, underestimates PC. 18 F-FDG PET/CT shows instead 90% sensitivity and preoperative specificity of 77% for degree II and III lesions; nevertheless it underestimates small lesions in all locations, when the size of the nodules is less than 5 mm. In the follow-up period, CT can detect early recurrences, even if a differential diagnosis between true relapses and fibrotic scars caused by treatments is often difficult; in these patients 18 F-FDG PET/CT can help to make a differential diagnosis.
Usually 18 F-FDG PET/CT is positive after completeness of cytoreduction index grade 2 (CC2) to CC3 and negative after CC0 to CC1.
Not all histologic types show good glucose uptake at 18 F-FDG PET/CT; it could therefore be advisable to perform a preoperative examination to assess the initial glucose uptake to exclude false negatives in the follow-up period of nonuptaking tumors. Recent studies showed that both CT and 18 F-FDG PET/CT were unable to give a correct staging of carcinomatosis. One may conclude that nowadays there is no noninvasive procedure that can correctly evaluate PCI and expected cytoreduction index after treatment, especially if the lesions are small ( Figs. 1 and 2 ).
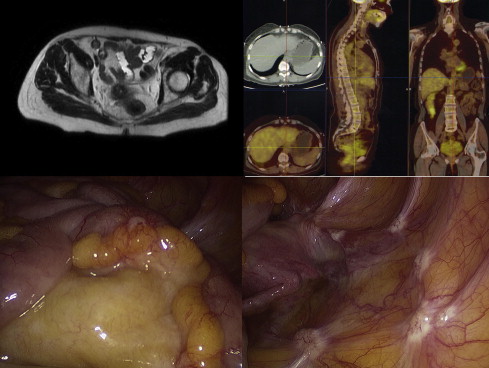
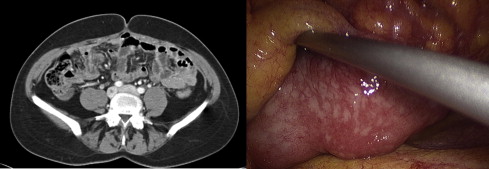
Indications for integrated treatment: locoregional staging
The first attempt at defining locoregional staging of peritoneal carcinomatosis was conducted by the Japanese, after the first studies completed in the 1990s about the association of surgery and locoregional chemotherapy for the treatment of locally advanced gastric cancer.
A staging format for carcinomatosis from gastric cancer was proposed:
- •
P1 (few nodules above the mesocolon)
- •
P2 (moderate amount of nodules even below transverse mesocolon)
- •
P3 (many spread nodules)
This classification is simple, shows a good prognostic value, with a 2-year survival rate that falls from 21% for P1 to 4% for P3 and, with slight modifications, can be used even for different forms of carcinomatosis; notwithstanding, it is not accurate enough concerning the distribution and localization of neoplastic lesions.
Gilly’s classification is also very simple, but more modern:
- •
Stage 0: no macroscopic signs of disease
- •
Stage I: nodules smaller than 5 mm, confined to one abdominal region
- •
Stage II: nodules smaller than 5 mm, disseminated through the abdomen
- •
Stage III: size of nodules between 5 mm and 2 cm
- •
Stage IV: lesions larger than 2 cm
Even so, this classification is not detailed enough about the distribution of the lesions.
Sugarbaker’s classification is more useful for both prognosis and research, and is based on the PCI ( Fig. 3 ). The abdomen is divided into 9 sectors and the small bowel into 4 more parts; for each sector a score is assigned (Lesion Size score [LS]) related to the actual disease:
- •
LS 0: no macroscopic evidence
- •
LS 1: maximum diameter of the lesions up to 0.5 cm
- •
LS 2: maximum diameter up to 5 cm
- •
LS 3: maximum diameter larger than 5 cm or confluent nodules
The total of the scores for all sectors gives the PCI.
Stage P1 to P2 of the Japanese classification is equivalent to Gilly’s Stage I to II and to Sugarbaker’s PCI of less than 13.
The expected achievement of CC0 remains, however, the main prognostic factor : patients who achieve CC1 cytoreduction have a markedly worse outcome, whereas those classified as CC2 to CC3 show very poor results.
If one has to consider Peritonectomy + HIPEC as a curative treatment of peritoneal carcinomatosis, patients who cannot be classified as expected CC0 should be excluded from the procedure. CC1 cases (residual lesions between 0.25 and 2.5 mm) in HIPEC-responder patients can also be considered CC0 after cytoreduction. In CC1 HIPEC nonresponders, CC2, and CC3, the integrated treatment offers limited increase in OS but a marked improvement in quality of life; it can therefore be considered as advanced palliative surgery.
Histology of the primary tumor plays a crucial role in the selection of patients affected by peritoneal carcinomatosis, because it noticeably modifies the cutoff value of PCI during the decision-making process. Selection of patients cannot therefore leave out of consideration the following factors:
- •
PCI
- •
Histology of the primary tumor
- •
Expected CC index
These factors must be related to:
- •
Age
- •
Karnofsky Index
- •
Comorbidities (cardiovascular, respiratory)
- •
Carcinomatosis-related complications at the time of surgery (intestinal obstruction, ascites)
- •
Active infections
- •
Previous systemic chemotherapy, chemoresistance, toxicity
- •
Disease-free interval from previous surgery
Previous evaluation of resectable hepatic metastases and of possible involvement of other organs, such as pancreas, is mandatory, because an eventual pancreatic resection greatly increases morbidity.
Infiltration of small bowel and its mesentery by lesions that cannot be reduced to CC0 even after HIPEC (and therefore requiring surgical resection) can be treated, providing the remaining healthy small-bowel length is at least one-third of the total.
Role of staging laparoscopy
Video-laparoscopy (VLS) is considered an excellent diagnostic procedure, but its wide application in peritoneal carcinomatosis has been discouraged, the objections to this technique being related to:
- •
Difficulty of trocar positioning in the presence of abdominal wall tumor masses or adhesions from previous surgery
- •
Skepticism about the reliability and efficacy of the procedure in the staging phase
- •
Fear of neoplastic contamination of the port sites, supported by the finding of 52% of recurrences for pseudomyxoma peritonei along the surgical scar
Notwithstanding the aforementioned considerations, in their extensive experience the authors have documented a possibility of trocar positioning near 100% (even in patients with multiple previous surgery), the absolute reliability of VLS staging, and the complete absence of neoplastic contamination of the abdominal wall around the port sites, if trocars are inserted using a standardized technique.
Since the beginning of their experience in 2000, the authors have related the information from VLS staging with PCI with the possibility of obtaining complete cytoreduction. Using this methodology, almost one-half of the patients were excluded from the treatment, many of them being submitted to a second staging laparoscopy after systemic neoadjuvant chemotherapy.
Laparoscopy in the staging of peritoneal carcinomatosis
Main aim of laparoscopic staging is to carefully assess the patient’s prognosis and the surgical feasibility, considering that the simple surgical exploration is often dangerous in such patients: in advanced abdominal tumors treated with laparotomy alone morbidity from 12% to 23% and mortality from 20% to 36% have been reported.
Diagnostic imaging (CT and CT/PET) is still considered the first and mandatory diagnostic test for peritoneal carcinomatosis: when imaging-based PCI is in favor of enrolling the patient for treatment, VLS staging allows assessment of the true PCI, granting a correct selection of patients according to the expected CC index and a cost/benefit assessment in terms of DFS, OS, and quality of life.
Surgical Technique
The Hasson trocar is introduced and the ascites completely sucked out of the peritoneal cavity, taking care not to contaminate the port sites.
Considering the high incidence of adhesions both from previous surgery and from tumor masses infiltrating the midline, the choice is to avoid median or paraumbilical access; the authors prefer a trocar positioned in the right or left flank or iliac fossa on the mid-axillary line after carrying out clinical evaluation and ultrasound scan of the considered quadrants.
This access allows for a better exposure of the small bowel and its mesentery even in presence of a large omental cake; it also offers the possibility of improving visualization by inserting a second trocar (5 mm) beneath the first one or in the contralateral iliac fossa.
A 30° scope is routinely used; division of adhesions should be kept to a minimum to avoid the risk of lesions to abdominal organs, but should be enough for a complete evaluation of the PCI. In case of tenacious adhesions or neoplastic infiltrations of the median line, it is advisable to explore the right and left sections separately and to carry out a second open access to view the quadrants of the opposite side.
Cytology samples should be taken under direct view. Highly mucinous carcinomatosis sometimes requires a 10-mm trocar in port II to admit insertion of a larger suction cannula. In peritoneal surface malignancies where the pathologic findings are unknown or doubtful, it is important to collect multiple biopsy specimens from the parietal, omental, and pelvic cavity lesions. Diaphragmatic biopsies can cause perforation and infiltration of the muscular wall and should be avoided. When liver metastases or the involvement of major hepatic veins are suspected, as in diaphragmatic lesions larger than 2 cm, VLS ultrasound imaging can be helpful.
To accomplish the laparoscopic definition of PCI, which is determined on the basis of the distribution and size of the tumor nodules, the operating table has to be moved into at least 4 positions: steep anti-Trendelenburg left tilt, steep anti-Trendelenburg right tilt, steep Trendelenburg left tilt, and steep Trendelenburg right tilt.
Personal Experience and Results
The authors’ group performed 351 diagnostic VLS procedures in patients with peritoneal surface malignancies ( Table 1 ). The average time needed for a diagnostic and staging VLS procedure was 30 minutes (range 15–45 minutes). In one patient the access to the abdominal cavity was impossible because of thick cancerous adhesions between the small-bowel loops and the abdominal wall: this patient underwent midline laparotomy, which confirmed the impossibility of reaching into the abdominal cavity because of massive involvement of the small-intestine loops tightly adherent to the abdominal wall.
| Pathology | Cases | Diagnostic | Unfeasible | Understaging | 2-Trocar | 3-Trocar | Site Infection | Bleeding | Bowel Perforation | Diaphragm Perforation |
|---|---|---|---|---|---|---|---|---|---|---|
| Ovary | 121 | 121 | 2 | 120 | 1 | 1 | ||||
| Stomach | 76 | 75 | 1 | 1 | 70 | 8 | 1 | 1 | ||
| Colon-rectum | 73 | 73 | 71 | 3 | 1 | 1 | ||||
| Appendix cancer | 24 | 24 | 1 | 24 | ||||||
| Breast | 15 | 15 | 15 | |||||||
| Mesothelioma | 14 | 14 | 1 | 14 | ||||||
| Pancreas | 10 | 10 | 1 | 9 | 1 | |||||
| Uterus | 8 | 8 | 8 | |||||||
| Sarcoma | 6 | 6 | 6 | |||||||
| DIRCT | 2 | 2 | 2 | |||||||
| Prostate | 1 | 1 | 1 | |||||||
| Duodenum | 1 | 1 | 1 | |||||||
| Total | 351 | 350 | 1 | 5 | 333 | 21 | 2 | 1 | 1 | 1 |
| % | 100 | 99.72 | 0.28 | 1.42 | 94.87 | 6.02 | 0.57 | 0.28 | 0.28 | 0.28 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree