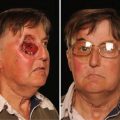
Fig. 11.1
Acute toxicity relative risk values (T RR) and relative max-grade values for 13 head and neck treatment groups ranked by increasing relative risk. P platinum, H hydroxyurea, 5-FU 5- fluorouracil, PA paclitaxel (From Trotti et al. [21], reproduced with permission)
Patient Factors in Concurrent Chemoradiotherapy
As mentioned in Chap. 6 (Clinical decision making), there are several factors playing a role in decision making during multidisciplinary team meetings, i.e., disease factors, patients factors, treatment factors, and adequate communication with and information to the patient, giving sufficient support, taking into account the wish of the patient. With respect to patient factors, we mentioned that among others age, sex, performance status, nutritional status, comorbid chronic disease, oral health, lifestyle habits, and socioeconomic status all play a role. Multiple studies have shown several demographic and health status characteristics to be associated with a higher chance of noncancer-related death [22–26]. Factors most commonly associated with increased noncancer-related mortality include increasing age, male sex, increasing comorbidity, decreasing body mass index (BMI), and an unmarried status. In a cohort study of 479 patients with stage III/IV carcinoma of the head and neck, all treated with CCRT, with or without induction chemotherapy, in a series of five successive multi-institutional protocols in the USA, the authors reported results of a multivariable analysis on predictors of competing mortality. The study showed that older patients with comorbidities were more likely to die of noncancer causes, while women and patients with a lower BMI (log BMI lower than 3) and traveling shorter distances to the treating center were at higher risk for treatment-related mortality [23]. Median distance traveled correlated with race (blacks 5 miles, nonblacks 27 miles, p < 0.001), nevertheless race was not considered to be a confounding factor. In the decision making on how and with what to treat the patients, his/her own wish is playing a crucial role. It is therefore important to be aware of the considerations and the prioritizations of the head and neck cancer patient. In that regard, the study reported by List et al. [27] is of interest. Two hundred forty-seven newly diagnosed head and neck cancer patients from nine institutions in the USA and 131 nonpatients were asked to rank a set of 12 potential treatment outcomes from highest [1] to lowest [12]. The top three items most frequently mentioned by both patients and nonpatients were “being cured of cancer,” “living as long as possible,” and “having no pain,” in that order. In contrast, head and neck cancer patients ranked “cure” (90 % vs. 80 %) more frequently, while they ranked “no pain” less frequently (34 % vs. 52 %) in the top three. So, evidently for patients, survival seems to be of paramount importance, overshadowing associated toxicities and potential dysfunction. This means that unless there are unsurmountable objections we should choose for the best treatment approach. However, alternative options should be mentioned and discussed, balancing pros and cons of each treatment option within the context of that individual patient.
If indeed high-dose cisplatin-based CCRT is the preferred standard of care treatment option for patients with LA-SCCHN, one should be aware of the absolute and relative contraindications of that treatment. According to experienced radiation oncologists, there are no absolute contraindications for radiotherapy, although extreme caution should be given to considerations of radiotherapy for patients with scleroderma (especially those with CREST [Calcinosis, Raynaud phenomenon, Esophageal dysmotility, Sclerodactyly, and Teleangiectasia] syndrome) and ataxia telangiectasia. Moreover, caution is also generally advised in case of Fanconi’s anemia, systemic lupus erythematosis, rheumatoid arthritis, dermato- and polymyositis, and other autoimmune and collagen vascular conditions (Brian O’Sullivan, personal communication). It goes without saying that re-irradiation is also a major concern in all patients, and is addressed more specifically elsewhere in this textbook. With respect to the use of cisplatin, absolute and relative contraindications for the use of cisplatin in general and high-dose cisplatin in particular were defined by a consensus panel in August 2014 [28]. Absolute contraindications for the use of (high-dose) cisplatin include an ECOG score ≥3, a creatinine clearance < 50 ml/min, preexisting hearing loss or tinnitus ≥ grade 2, neurologic disorders ≥ grade 2, known hypersensitivity to platinum-based therapy, pregnancy and lactation, and HIV/AIDS (with CD4 count < 200/μl). Relative contraindications for the use of cisplatin are summarized in Table 11.1.
Table 11.1
Clinical criteria for patients at high risk for platinum toxicity. ECOG Eastern Oncology Cooperative Group, NCI-CTC National Cancer Institute – Common Toxicity Criteria, HIV/AIDS human immunodeficiency virus infection/acquired immune deficiency syndrome, CD4 cluster of differentiation 4
ECOG performance status 2 |
Biological age (>70 years; geriatric assessment, cognitive function) |
Creatinine clearance 50–60 ml/min (consider lower dose of cisplatin) |
Borderline function (NCI-CTC grade 1) of target organs (oto/neuro) |
Marrow, hepatic, and respiratory dysfunction ≥ grade 2 (Child-Pugh score B) |
Comorbidities (cardiovascular, diabetes, recurrent pulmonary infections) |
HIV/AIDS (CD4 count < 350/μl)/immune-compromised conditions |
Previous cisplatin therapy, including induction chemotherapy (>200 mg/m2) |
Involuntary weight loss (≥20 %) and a low body mass index |
Concomitant use of nephrotoxic drugs |
Socioeconomic status; lack of social support, no support at home |
Treatment Factors in Concurrent Chemoradiotherapy
Methods to reduce the toxicity of cisplatin-based CCRT include, among others, better radiation targeting, use of newer RT techniques, and alternatives to the use of high-dose cisplatin.
Better Targeting and New Radiotherapy Techniques
Better targeting can be obtained by computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) scans and more accurate delivery using daily image-guided radiotherapy. More precise contouring reduces toxicity as it lowers the volume of unnecessary irradiated healthy tissue and increases tumor control by reducing the risk of geographical misses. New radiation techniques such as rotational intensity-modulated radiotherapy (IMRT) has gradually replaced the static beam IMRT, leading to a more conformal dose distribution and better sparing of organs at risk [29]. IMRT became state of the art in head and neck cancer therapy based on level I evidence of static beam IMRT reducing xerostomia compared to conventional radiotherapy, while evidence of superiority of IMRT to conventional radiation both in terms of quality of life and survival is growing [30–37]. In a Dutch study, grade ≥2 swallowing dysfunction in head and neck cancer patients treated with (chemo) radiation was best predicted by the mean radiation dose to the superior pharyngeal constrictor muscle and to the supraglottic larynx and swallowing sparing IMRT therefore seems of interest for specific patient categories [38]. Parallel to IMRT, there has been the clinical implementation of stereotactic radiation (CyberKnife and linac-based stereotactic RT) and particle therapy (protons and carbon ions). Stereotactic radiotherapy or radiosurgery is a nonsurgical procedure that delivers targeted irradiation very precisely at much higher doses than conventional radiotherapy while sparing the nearby organs at risk. Stereotactic radiotherapy in head and neck cancer can be used as a boost after conventional radiation or for re-irradiation after relapse or second primary in previous irradiated areas. As it is felt by some that photon-based radiotherapy may have reached the limits of its possibilities, other particles gained interest. Most popular are protons and carbon ions. Both have dose distributions that are superior to any photon technique. This approach may be particularly interesting for children and when there are radiosensitive organs at risk nearby, such as spinal cord, brainstem, parotid, and submandibular glands. Carbon ions will mainly be used for radioresistant cancers because of its supplementary higher biologic effect (e.g., melanoma, adenoid cystic carcinoma, certain sarcoma subtypes).
Alternatives for High-Dose Cisplatin (in Combination with RT)
Several alternatives for the use of high-dose cisplatin can be considered, such as (1) other cisplatin doses or schedules, (2) other cytotoxics, e.g., carboplatin, taxanes, or low-dose gemcitabine, (3) targeted agents, e.g., cetuximab (the only approved targeted agent for head and neck cancer), or (4) hypoxic modification (an approach which is used in standard [chemo] radiation schedules in Denmark).
Many attempts have been made to reduce the toxicity of cisplatin-based CCRT while maintaining the antitumor effect. Parameters of cisplatin that theoretically can be modified are the cumulative dose, the dose intensity, the peak dose, and the administration schedule. In a recent systematic review, Strojan et al. [39] concluded, based on six definitive chemoradiation trials, that there is a statistically significant association between treatment outcome and the cumulative dose of cisplatin, independent from the schedule used, showing survival benefit for the higher doses. Although the critical cumulative dose is not exactly known, earlier publications suggested to use minimally a cumulative dose of 200 mg/m2 [40–42]. That cut-off point was used in a retrospective study of 659 newly diagnosed patients with LA-SCCHN (404 HPV-positive/95 % OPC; 255 HPV-negative/38 % OPC) treated with cisplatin-based CCRT at the Princess Margaret Cancer Center in Toronto, Canada, and the Istituto Nazionale dei Tumori in Milan, Italy, from 2000 till 2012. Overall survival was significantly less in patients treated with a cumulative dose below 200 mg/m2 compared with those receiving higher cumulative doses. However, that only was the case in patients with HPV-negative tumors. No such difference in outcome was observed in patients with HPV-positive tumors [43]. This could be in agreement with the observed higher sensitivity of HPV-positive tumors to both radiation and chemotherapy and give support to the ongoing studies testing the concept of deintensification in HPV-positive OPC patients. Many cisplatin-induced toxicities are peak dose related; therefore administering the 100 mg/m2 cisplatin dose over a longer period of time (e.g., 24 h) or giving the dose split over 5 days might be an option that could induce less toxicity. Although this has not been explored in head and neck cancer patients in a prospective randomized study, the toxicity data reported of the German ARO 96–3 trial, a study in which CCRT with cisplatin (20 mg/m2/d × 5) plus 5-FU was compared to RT alone, do suggest that might be a reasonable option [44]. Although there is no evidence of its equivalence with the high-dose cisplatin regimen, there is a tendency to use a weekly low-dose cisplatin (40 mg/m2) in several areas of the world. One may argue that more frequent cisplatin administrations during RT might lead to stronger radiosensitization and less chance of radioresistance. However, some caution is needed since recent data presented at ASCO 2015 showed that survival data might be less with that approach [45]. Comparative studies are therefore needed before the low-dose weekly regimen is to be adopted as the new standard approach of administering cisplatin in CCRT strategies.
The use of other cytotoxics has mainly been studied in patients that were not candidates for cisplatin or showed unacceptable toxicity when they started on cisplatin. Carboplatin has a more favorable toxicity profile with lower rates of otoxocity, nephrotoxicity, neurotoxicity, and emesis [46]. Carboplatin is primarily excreted with the urine and therefore can be better dosed based on the glomerular filtration rate [47]. There are no large randomized trials comparing carboplatin versus cisplatin in the CCRT setting, and the individual patient-based meta-analysis from Pignon et al. [11] suggested that monochemotherapy with drugs other than cisplatin led to inferior results and therefore should not be recommended in routine practice. Therefore, despite the fact that more contemporary studies suggest that it might be a reasonable option when cisplatin is contraindicated or not tolerated [48], adequate trials supporting this notion are needed. The same can be said about taxanes, although some data on CCRT in the postoperative setting suggested a beneficial effect of taxanes versus cisplatin. In RTOG 0234, 238 patients were randomized to receive 60 Gy radiation with cetuximab once weekly plus either cisplatin 30 mg/m2 or docetaxel 15 mg/m2 once weekly. The 2-year disease-free survival (66 % vs. 57 %) and 2-year overall survival (79 % vs. 69 %) were in favor of the taxane arm [49]. Further studies in that direction seem appropriate. A recent review highlighted the enormous radiosensitizing potential of gemcitabine and suggested that very low dosages (less than 50 mg/m2 per week) provide a sufficient therapeutic ratio and therefore should be further investigated [50]. However, many of the reported studies lack sufficient data on late toxicity. For those without any experience with gemcitabine in this clinical setting, it is not advised to use it outside clinical trials.
The addition of cetuximab to irradiation improves locoregional control and prolongs progression-free survival and overall survival [51, 52]. Treatment adherence of >90 % of cetuximab plus RT seems better than what has been observed with cisplatin-based CCRT, and quality of life with cetuximab plus RT was not found inferior to that with RT alone [53]. Despite these promising data, many clinicians in Europe are still hesitant to use cetuximab plus RT routinely instead of platinum-based CCRT, because of lack of large prospective randomized phase III trials that compare efficacy and adverse events of RT plus cetuximab versus RT plus cisplatin (or RT plus carboplatin/infusional 5-FU for that matter, both considered standard of care CCRT regimens). Most clinicians see RT plus cetuximab as a treatment option for patients with absolute (or relative) contraindication for platinum-based therapy. A further trigger to this discussion was evoked by a recent small randomized phase II study with a rather negative outcome of the bioradiation arm [54]. For further reading on this topic, the reader is advised to read the two editorials that appeared in relation to this article [55, 56].
Hypoxic modification is another biological modification that has markedly improved outcome [4]. Nimorazole is the most studied hypoxic radiosensitizer. Previous studies in Denmark have shown that nimorazole improves outcome of radiation, whether given by conventional fractionation or accelerated fractionation, without an indication that the (late) complication rate of the radiotherapy thereby is increased. The positive effect on locoregional control and survival was particularly evident in the more strongly hypoxic tumors and less so in the less hypoxic tumors. HPV/p16 positive tumors did not seem to benefit from hypoxia modification, despite the fact that they may express hypoxic features. The feasibility and tolerance of the combined schedule of nimorazole, accelerated fractionation radiotherapy, and CCRT with weekly cisplatin (40 mg/m2 weekly for at least 5 weeks) has been evaluated in the DAHANCA 18 study and since then has become standard in Denmark. These intriguing data ask for confirmation, and this will hopefully be done by protocol 1219 ROG-HNCG of the EORTC, comparing CCRT plus nimorazole versus CCRT plus placebo. This study will be executed outside Denmark. The two primary endpoints of the trial are: (1) to study whether locoregional control rate can be improved with this combined approach and (2) to test whether the benefit is restricted to patients whose tumors have a hypoxic gene profile. In case confirmation of the Danish study can be obtained, this will lead to new treatment possibilities and opportunities.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree