Unlike neuropathic pain associated with diabetes, which starts in the feet and spreads to the hands over months or years, neuropathic pain caused by chemotherapy often begins in the feet and the hands acutely.
Presentation is predominantly dose-dependent sensory symptoms (especially pain) in both frequency and severity, rather than motor symptoms.
There exists a length-dependent “dying back” distribution, with the earliest symptoms occurring at fingertips and toes, followed by a progression of symptoms proximally along the limbs as the neuropathy progresses. This pattern of CIPN has been attributed to the fact that the longest fibers have the greatest surface area exposed to CIPN drugs and hence are at risk for greater toxicity.
Known onset and extent of neuronal or nerve injury induced by chemotherapeutic drugs provides an opportunity for translational work through both basic and clinical research to test preemptive trials for CIPN.
Histological findings have indicated that, unlike painful peripheral neuropathies due to trauma and diabetes, CIPN-related pain occurs in the absence of axonal degeneration in peripheral nerves.
TABLE 6.1 Characteristics of peripheral neuropathy induced by common chemotherapeutic agents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
of the myelin sheath, leading to a decrease in nerve conduction velocity (16). Vincristine-induced neuropathic pain is characterized by abnormal spontaneous discharges in the A-fiber and C-fiber primary afferent neurons (17), but pain is not a prominent feature of vincristine-induced peripheral neuropathy (18).
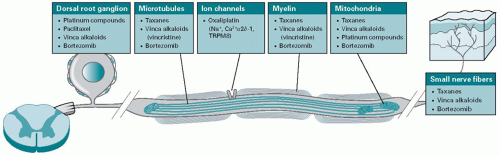 Figure 6.1. A proposed diagram illustrating the targets of most commonly used chemotherapy-induced neurotoxicity in the peripheral nervous system. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree