Fig. 3.1
Peripheral blood (PB) film from a patient with chronic myelogenous leukemia with an unusually high white cell count showing an increase of neutrophils, eosinophils, basophils, and granulocyte precursors. May-Grünwald-Giemsa stain (MGG), high power
Peripheral blood features of the accelerated phase of CML may include: leucocytosis or thrombocytosis that is refractory to treatment; an increasing basophil count; a disproportionate increase in blast cells; the appearance of dysplastic features such as hypolobulated neutrophils and circulating micromegakaryocytes; thrombocytopenia; and increasing anemia. A disproportionate increase in eosinophils can occur but is much less common than marked basophilia. Poikilocytosis may become more marked and there may be teardrop poikilocytes.
In blast transformation there is usually an increase in blast cells in the peripheral blood (Fig. 3.2). Usually these are myeloblasts, megakaryoblasts, or lymphoblasts. When transformation is megakaryoblastic, there may also be circulating micromegakaryocytes. Less common forms of transformation (determined by the underlying further mutations that have occurred) include monoblastic, eosinophilic, erythroblastic, and hypergranular promyelocytic.
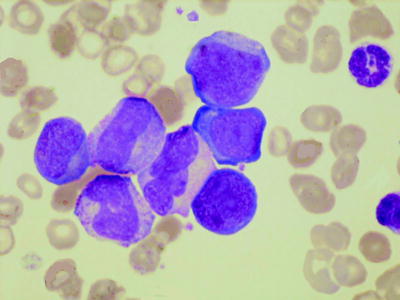

Fig. 3.2
PB film from a patient with transformation of chronic myelogenous leukemia showing three blast cells, granulocyte precursors, and several dysplastic cells of neutrophil lineage. MGG, high power
Bone Marrow Cytology
The bone marrow aspirate in chronic phase CML shows marked hypercellularity due to an increase in granulocytes and their precursors (Fig. 3.3). The myeloid:erythroid ratio is almost always greater than 10:1 and often of the order of 25:1. Megakaryocytes are usually increased with a tendency to be smaller than normal with reduced nuclear lobulation, reflecting a decrease in ploidy. However, micromegakaryocytes, such as those seen in the myelodysplastic syndromes, are not a feature of chronic phase disease. Sometimes there is an increase in storage cells—pseudo-Gaucher cells and sea blue histiocytes.
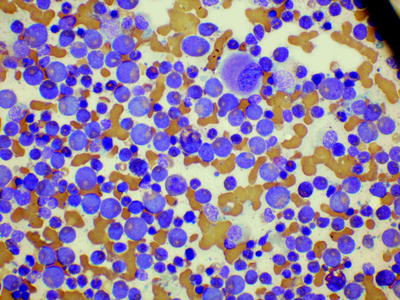

Fig. 3.3
Bone marrow film from a patient with chronic myelogenous leukemia showing increased granulopoiesis and a hypolobated megakaryocyte. MGG, low power
In the accelerated phase, the aspirate may show increased blast cells, increased basophils, and dysplastic features in the cells of any lineage.
In blast transformation, the bone marrow shows increased blast cells, except in the minority of cases in which transformation is first detected at an extramedullary site. Myeloblasts are often agranular and Auer rods are usually absent. In the case of myeloid or mixed lineage transformation, there are usually dysplastic features, particularly in the megakaryocyte lineage (Fig. 3.4).
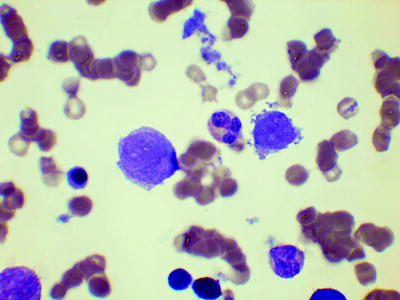

Fig. 3.4
Bone marrow film from a patient with megakaryoblastic transformation showing a neutrophil flanked by a blast cell (left) and a micromegakaryocyte (right). MGG, high power
Flow Cytometric Immunophenotyping
Immunophenotyping is not diagnostically useful in chronic phase disease.
Histology
In chronic phase CML, trephine biopsy sections show an increase in cells of all granulocyte lineages but, apart from the loss of fat cells, with retention of normal bone marrow architecture (Fig. 3.5) [5–7]. There is an expansion of the band of myeloblasts and promyelocytes that is usually detected against the bony spicule and around arterioles. Eosinophils are readily detected, but basophils are not specifically identifiable on hematoxylin and eosin (H&E) stains as granules are dissolved during processing. Megakaryocytes are increased in number with a reduction in average size and nuclear lobulation. They are normally located and do not form large clusters. Erythropoiesis is decreased. Mast cells and plasma cells are often increased. Increased storage cells may be apparent (Fig. 3.6). Bone marrow vascularity is increased (neoangiogenesis). Reticulin is usually normal or only mildly increased.
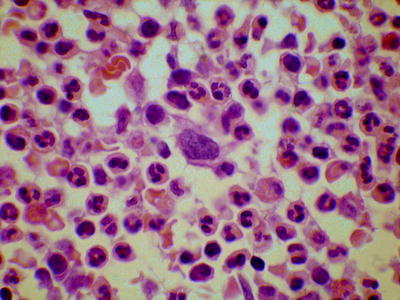
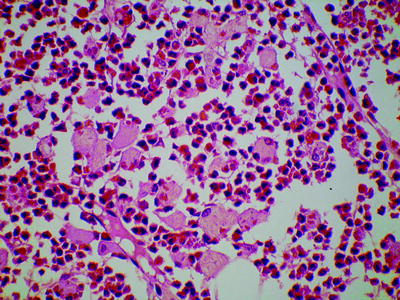

Fig. 3.5
Bone marrow trephine biopsy section from a patient with chronic myelogenous leukemia showing increased granulopoiesis and a relatively small, hypolobated megakaryocyte. Hematoxylin and eosin stain (H&E), low power

Fig. 3.6
Bone marrow trephine biopsy section from a patient with chronic phase chronic myelogenous leukemia showing numerous pseudo-Gaucher cells. H&E, low power
In accelerated phase, the changes that would be expected from the bone marrow aspirate are present. In addition, the bone marrow architecture may be abnormal, e.g., with megakaryocytes located adjacent to bony spicules or forming large clusters. Intravascular hemopoiesis and bone marrow necrosis can occur. Reticulin may be increased. Sometimes the increase in reticulin is marked and there is also collagen fibrosis and osteosclerosis (Fig. 3.7).
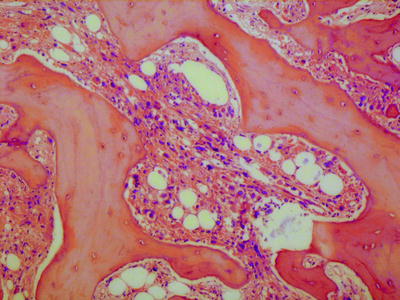

Fig. 3.7
Bone marrow trephine biopsy section from a patient with accelerated phase of chronic myelogenous leukemia showing myelofibrosis and osteosclerosis. There are numerous dysplastic megakaryocytes embedded in the loose fibrous tissue. H&E, low power
Immunohistochemistry with CD42b or CD61 monoclonal antibodies can be used to identify dysplastic megakaryocytes. CD34 antibodies can identify blast cells and the endothelial cells of new vessels.
In blast transformation, there is an increase in the blast cells of one or more lineages. In myeloid transformation, there can also be a marked increase of dysplastic megakaryocytes, often in large clusters or sheets. The pattern of blast infiltration may initially be random focal, but subsequently blast cells obliterate maturing hematopoietic cells. Reticulin and collagen fibrosis are common, particularly when there is an increase in megakaryoblasts and dysplastic megakaryocytes.
Immunohistochemistry can be used to identify myeloblastic crisis (CD68, lysozyme), megakaryoblastic crisis (CD42b, CD61), erythroblastic crisis (antiglycophorin—CD235a or CD236R), and B-lymphoblastic crisis (CD79a is more generally positive than CD20). CD34 immunohistochemistry can help in the quantification of blast cells.
In extramedullary transformation, there is initially infiltration of another tissue or organ, e.g., a lymph node, with subsequent spread to the marrow. In extramedullary transformation, immunohistochemistry is useful for confirmation of the diagnosis.
Atypical (Ph-Negative) Chronic Myeloid Leukemia
Atypical chronic myeloid leukemia (aCML) is an uncommon, Ph-negative, BCR-ABL1-negative chronic myeloid leukemia, which is categorized in the WHO classification as an MDS/MPN [8–10]. Clinical features are similar to those of CML, but the prognosis is worse. Death may result from bone marrow failure or evolution to acute myeloid leukemia (AML). Atypical CML is mainly a disease of adults, particularly elderly adults with a similar incidence in men and women
Peripheral Blood Count and Cytology
The peripheral blood shows leukocytosis and anemia (Fig. 3.8). There may be anisocytosis, poikilocytosis, macrocytosis, or dimorphism. The platelet count is often reduced but may be normal or increased. In comparison with CML, anemia tends to be more severe and thrombocytopenia more common. There is an increase in neutrophils and their precursors. Eosinophils and basophils are often increased but less consistently than in CML. However, some patients have prominent eosinophilia. The monocyte count is relatively higher than in CML; it may be more than 1 × 109/l, but monocytes are not usually more than 10 % of leucocytes. Granulocyte precursors are also present. In comparison with chronic myelomonocytic leukemia, promyelocytes, myelocytes, and metamyelocytes are at least 10 % of leucocytes and sometimes 15 % or higher. These may include blast cells but, by definition, blast cells (plus promonocytes) are less than 20 % in the blood (and the bone marrow). Dysplastic features are present in neutrophils; hypolobation, abnormal nuclear shapes, increased chromatin clumping, and reduced granularity may be seen. Monocytes may also be dysplastic, showing hyperlobation or hypolobation, increased cytoplasmic basophilia, and increased granularity. The neutrophil alkaline phosphatase score is variable and is not diagnostically useful.
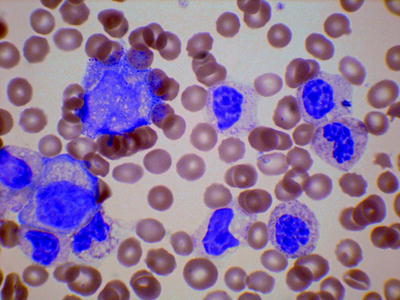

Fig. 3.8
PB film from a patient with atypical chronic myeloid leukemia showing neutrophil precursors and dysplastic neutrophils (hypolobated, nuclear clumping, and hypogranularity). MGG, high power
Bone Marrow Cytology
The bone marrow aspirate shows increased cellularity with an increase mainly in neutrophils and their precursors. Monocytes and their precursors may be increased and a nonspecific esterase stain can help in their detection. Megakaryocytes may be present in normal numbers or may be decreased or increased. There is dysplasia, which is often of trilineage. Dysgranulopoiesis is usual, but there may also be ring sideroblasts and other features of dyserythropoiesis, hypolobated megakaryocytes, and micromegakaryocytes.
Flow Cytometric Immunophenotyping
Immunophenotyping is not known to be diagnostically useful.
Genetics
Karyotypic abnormalities are common and can include trisomy 8, 20q–, i(17q) and abnormalities of chromosomes 12, 13, 14, 17, and 19. NRAS. KRAS, CBL, and TET2 may be mutated. Patients with BCR-ABL1 or rearrangement of PDGFRA, PDGFRB, or FGFR1 are specifically excluded from this diagnostic category.
Histology
Cellularity is increased as a result of an increase in neutrophils and precursors and a variable increase in monocyte precursors. There is dysplasia and the architecture is disorganized. Reticulin may be increased and collagen fibrosis and osteosclerosis occasionally occur. Immunohistochemistry is useful to highlight dysplastic megakaryocytes (CD42b and CD61) and increased monocytes (CD14 and CD68R).
Chronic Myelomonocytic Leukemia
This is Ph-negative chronic myeloid leukemia that is categorized in the WHO classification as an MDS/MPN [11, 12]. The most prominent clinical features are anemia and splenomegaly, but skin and lymph node infiltration and pleural, peritoneal, and pericardial effusions can also be seen. It is mainly a disease of the middle aged and elderly and shows a male predominance. Transformation to AML occurs in up to a quarter of the patients.
Peripheral Blood Count and Cytology
The peripheral blood shows a normocytic or macrocytic anemia. Red cells are sometimes dimorphic. Leukocytosis is usual but not invariable. There is monocytosis with, by definition, a monocyte count of more than 1 × 109/l (Fig. 3.9). Neutrophils may be increased, normal, or decreased. Neutrophil precursors may be present but, in contrast to aCML, they are less than 10 % of the cells and usually less than 5 %. There may be small numbers of blast cells and promonocytes; by definition, they total less than 20 % of the cells, but they are usually much less. The number of blast cells (plus promonocytes) in the blood is of prognostic significance and the presence of 5 % or more leads to a classification as CMML-2. A minority of patients have prominent eosinophilia. The platelet count is often reduced but can be normal or high.
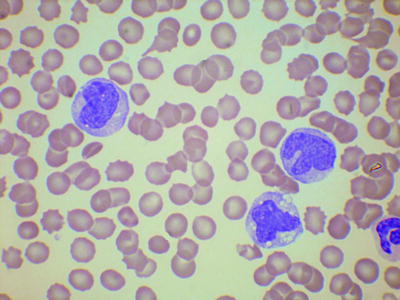

Fig. 3.9
PB film from a patient with chronic myelomonocytic leukemia showing thrombocytopenia and three immature, abnormal monocytes. MGG, high power
Dysplastic features may be present, but are usually less prominent than in aCML. Monocytes may be immature (cytoplasmic basophilia, reduced chromatin condensation, or reduced nuclear lobulation).
Bone Marrow Cytology
The bone marrow shows increased cellularity due to an increase of neutrophils and monocytes and their precursors but a nonspecific esterase stain may be necessary to demonstrate the increase in cells of monocyte lineage. Blasts plus promonocytes are less than 20 %. The number of blast cells (plus promonocytes) in the marrow is of prognostic significance and the presence of 10 % or more leads to the classification as CMML-2. Auer rods are rarely present but, when present, also lead to classification as CMML-2; a myeloperoxidase or Sudan black B stain is useful for their detection. Some patients have an increase of eosinophils and precursors. There is variable dysplasia, which may include ring sideroblasts.
Flow Cytometric Immunophenotyping
Immunophenotyping may show monocytes to be phenotypically abnormal with reduced, increased, or aberrant expression of various antigens. An abnormal phenotype may be the result of immaturity of monocytes (e.g., reduced CD14 expression) or of aberrant antigen expression (e.g., expression of CD2).
Genetics
Karyotypic abnormalities are detected in a quarter to a half of patients. They include trisomy 8, monosomy 7, del(7)(q) and rearrangements with a 12p breakpoint. Common molecular changes include mutations of RAS group genes, RUNX1, TET2, and CBL. By definition, BCR-ABL1 and rearrangement of PDGFRA and PDGFRB are absent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree