Fig. 4.1
Gross findings of ccRCC. The tumor is whitish yellow and well circumscribed, in which degeneration and hemorrhage are observed
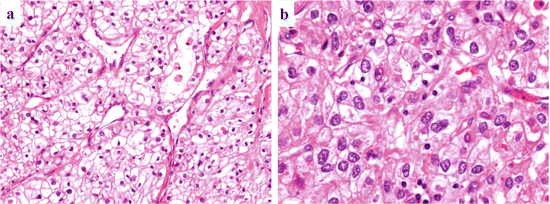
Fig. 4.2
Microscopic findings of ccRCC. Low-grade ccRCC has clear cytoplasm with a regular and delicate network of small thin-walled blood vessels (a), whereas high-grade ccRCC tends to have eosinophilic (granular) cytoplasm (b)
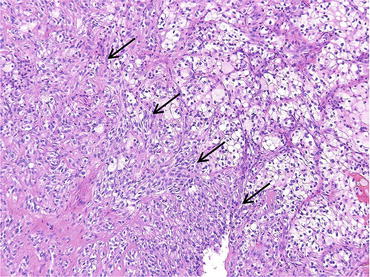
Fig. 4.3
Sarcomatoid carcinoma (arrows) in ccRCC
4.1.4 Grading
Nuclear grade is an important prognostic factor of ccRCC [15, 16]. There are 4-tiered and 3-tiered grading systems, and Fuhrman nuclear grade was most widely used (Fig. 4.4) [17]. However, the criteria for nucleolar prominence and nuclear pleomorphism are poorly defined in Fuhrman nuclear grade, and there is no indication regarding the relative importance of each feature. Therefore, the recently updated World Health Organization (WHO) classification recommends the use of WHO/International Society of Urological Pathology (ISUP) grading system [1]. According to WHO/ISUP grading system, nuclear grade 1 cells have small nuclei like mature lymphocytes, and nucleoli are absent or inconspicuous and basophilic at x400 magnification. Nucleoli of grade 2 cells are conspicuous and eosinophilic at x400 magnification and visible but not prominent at x100 magnification. The nucleoli of grade 3 cells are conspicuous and eosinophilic at x100 magnification. Extreme nuclear pleomorphism, multinucleated giant cells, and/or rhabdoid and/or sarcomatoid differentiation are observed in nuclear grade 4 cells. Generally, nuclear grade is assigned based on the highest grade present in ccRCC tissues.
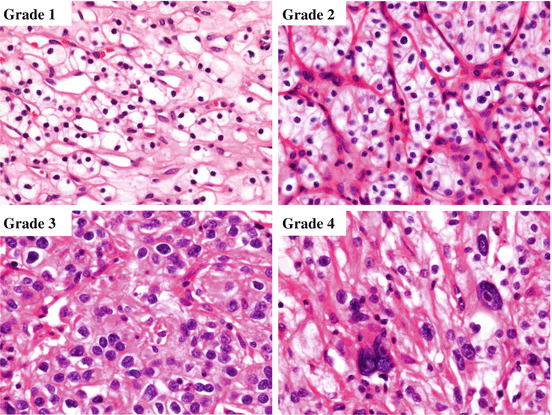

Fig. 4.4
WHO/ISUP grade. Grade 1 cells have small hyperchromatic nuclei resembling mature lymphocytes, and grade 2 cells have finely granular chromatin. The nucleoli can be easily recognized in grade 3 cells, and nuclear pleomorphism, hyperchromasia, and macronucleoli are observed in grade 4 cells
4.1.5 Immunophenotype and Differential Diagnosis
ccRCCs are diffusely positive for CA9 (Fig. 4.5), which is usually negative or focally positive in other renal tumors such as papillary and chromophobe RCCs and oncocytoma. Therefore, CA9 staining is helpful in the differential diagnosis of ccRCCs from other renal neoplasms. ccRCC is positive for low-molecular-weight cytokeratin (clone CAM5.2, CK18) and negative for high-molecular-weight cytokeratin (clone 34βE12, CK5/6). It is also positive for vimentin, EMA, RCC-Ma, CD10 (Fig. 4.5), PAX2, and PAX8 but usually negative for CK7, AMACR (α-methylacyl-CoA racemase), E-cadherin, and kidney-specific cadherin [18].
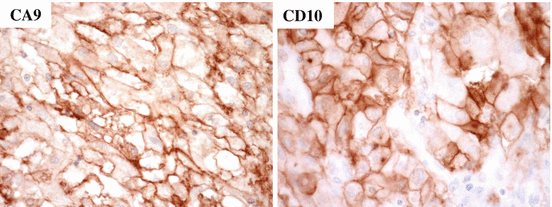

Fig. 4.5
CA9 and CD10 immunostaining in ccRCC
4.2 Multilocular Cystic Renal Neoplasm of Low Malignant Potential
4.2.1 General Features
Multilocular cystic renal neoplasm of low malignant potential is defined as a tumor composed of many cysts and fibrous septa containing small groups of clear cells without expansile growth, and these clear cells are indistinguishable from those of low-grade ccRCC [1]. Loss of heterozygosity of chromosome 3p was identified in most multilocular cystic renal neoplasm of low malignant potential by FISH analysis. VHL gene mutation was found in 25 % of multilocular cystic renal neoplasm of low malignant potential [19]. Recurrence or metastasis has not been reported [14].
4.2.2 Morphological Features
Grossly, multilocular cystic renal neoplasm of low malignant potential is a well-circumscribed tumor that is entirely composed of fibrous septa and numerous cysts (Fig. 4.6). RCCs containing a cystic structure and expansive nodules of tumor cells with clear cytoplasm must be diagnosed as ccRCC with cysts, not multilocular cystic renal neoplasm of low malignant potential (Fig. 4.7). This is because the patients with multilocular cystic renal neoplasm of low malignant potential showed excellent outcomes compared with those with ccRCC [20]. Microscopically, this tumor is composed of multiple cysts lined with tumor cells and fibrous septa (Fig. 4.8). The lining tumor cells have small dark nuclei and clear to pale cytoplasm, and they are flat or plump. By definition, the tumor cells do not form expansive nodules.
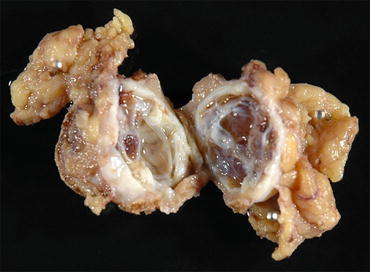
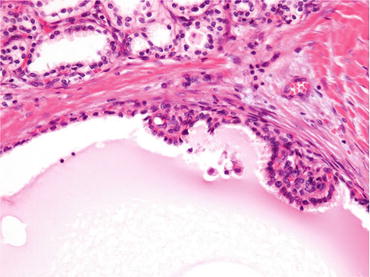
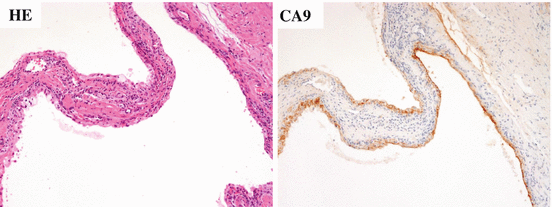

Fig. 4.6
Gross appearance of multilocular cystic renal neoplasm of low malignant potential

Fig. 4.7
Microscopic appearance of ccRCC with cysts. Cysts are observed in this case. However, this case should be diagnosed as cystic RCC with cysts, not multilocular cystic renal neoplasm of low malignant potential, because there are expansive nodules of tumor cells with clear cytoplasm

Fig. 4.8
Microscopic appearance of multilocular cystic renal neoplasm of low malignant potential. The tumor is composed of fibrous septa and tumor cells lining multiple cystic spaces without expansive nodules (a). Immunohistologically, tumor cells are positive for CA9 (b)
Immunohistochemically, cancer cells show positive staining for CA9 (Fig. 4.7), PAX2, PAX8, CD10, EMA, and CK7 but are negative for AMACR [19]. This tumor should be distinguished from other benign cystic renal neoplasms such as cystic nephroma, adult cystic nephroma/mixed epithelial and stromal tumor (MEST), and angiomyolipoma with epithelial cysts (AMLEC). Multilocular cystic RCC is positive for CA9, but other cystic tumors are negative for CA9 staining. Estrogen receptor (ER) and progesterone receptor (PgR) are negative in multilocular cystic RCC, whereas they are positive in adult cystic nephroma/MEST and AMLEC. Only AMLEC is reactive with antibodies for melanosome-associated antigen such as clone HMB45 and clone A103 (Melan-A).
4.3 Papillary RCC (pRCC)
4.3.1 General Features
pRCC is defined as a malignant renal tumor derived from renal tubular epithelium, and it has papillary or tubulo-papillary architecture [1]. Previously, any tumor with papillary and/or tubular architecture larger than 0.5 cm was classified as pRCC, while tumors 5 mm in diameter or smaller are defined as papillary adenomas [14]. However, unencapsulated WHO/ISUP low-grade (G1–2) tumors of papillary renal tumors ≤15 mm have virtually no capacity to metastasize and been classified as papillary adenoma, according to the recently updated WHO classification [1]. This tumor is the second most commonly encountered subtype of RCC and accounts for about 10 % of all renal tumors [21, 22], and the age, sex distribution, and signs of pRCC are similar to those of ccRCC [23, 24].
4.3.2 Morphological Features
Grossly, pRCC is well circumscribed and frequently contains areas of hemorrhage, necrosis, and cystic degeneration (Fig. 4.9) [23, 24]. Microscopically, pRCC is usually surrounded by a fibrous capsule and characterized by cancer cells forming papillary and papillo-tubular structures (Fig. 4.10). Foamy macrophages and cholesterol crystals in the fibrovascular core of the papillo-tubular structure are also characteristic features of pRCC. Necrosis and hemorrhage are frequently observed. Hemosiderin granules may be present in macrophages, stroma, and tumor cell cytoplasm [24]. pRCC has been subclassified into two categories, type 1 and type 2 pRCC, based on the morphologic features. In type 1 pRCC, cancer cells are small, and they have scanty basophilic or pale cytoplasm and low-grade nuclei without nuclear pseudostratification (Fig. 4.10a). Type 2 pRCC is composed of cancer cells having abundant eosinophilic cytoplasm and high-grade nuclei with pseudostratification (Fig. 4.10b). Sarcomatoid carcinoma is observed in about 5 % of pRCCs [25]. Importantly, papillary renal tumors showing features of other recognized morphological subtypes of RCC (i.e., MiT family translocation RCC, collecting duct carcinoma, mucinous tubular, and spindle cell carcinoma) should not be diagnosed as pRCC [1].
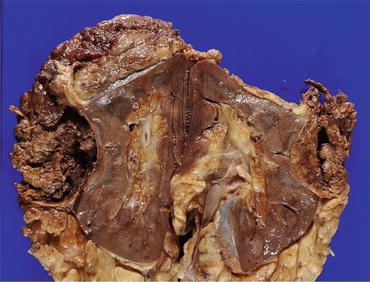
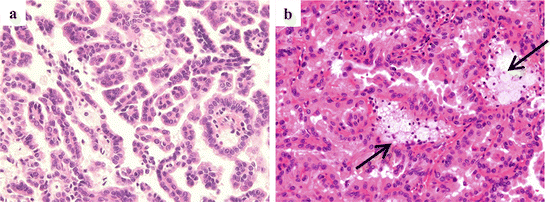

Fig. 4.9
Gross appearance of pRCC. The tumor is well circumscribed, and marked hemorrhage and necrosis are observed

Fig. 4.10
Microscopic appearance of pRCC type 1 (a) and type 2 (b). Arrows indicate foamy macrophages
Many pRCCs are positive for RCC-Ma, AMACR, PAX8, CD10, cytokeratin (CK) AE1/AE3, CK (CAM5.2), and CK7 (Fig. 4.11). pRCC is usually negative for high-molecular-weight CKs such as CK (34βE12), CK5/6, and TFE3 (Fig. 4.11). CA9 staining is negative or only focally positive in pRCC, in contrast to that in ccRCC, which shows diffuse CA9 staining. pRCC is associated with a more favorable prognosis compared to ccRCC and collecting duct carcinoma [1]. Generally, the prognosis of type 1 papillary RCC is better than that of type 2 tumors [26]. Sarcomatoid and rhabdoid differentiation is associated with poor survival, and the WHO/ISUP grading system is an important predictor for the patients with pRCC [1].
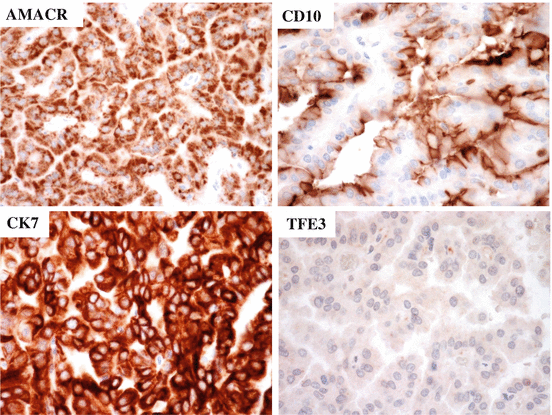

Fig. 4.11
Immunostaining of pRCC. Cancer cells are positive for AMACR, CK7, and CD10 but negative for TFE3
4.4 Chromophobe RCC (chRCC)
4.4.1 General Features
chRCC is defined as an RCC characterized by tumor cells with prominent cell membranes, wrinkled nuclei with perinuclear halos, and pale to eosinophilic cytoplasm, and it comprises about 5 % of all renal tumors [1]. The average patient age is in the sixth decade (range, 27–86 years), and both genders are equally affected. Many chRCCs are in stage T1 and T2 (86 %), and relatively few cases with metastatic disease have been reported [27].
4.4.2 Morphological Features
Grossly, chRCCs are well-circumscribed and solid tumors with slightly lobulated surfaces (Fig. 4.12). The cut surface of the tumor is homogeneously light brown or tan, and it turns gray after formalin fixation [14]. Microscopically, tumor cells tend to grow in solid or glandular patterns, with focal calcifications and broad fibrotic septa. The cell border is distinct, and wrinkled nucleus or binucleation is common. Perinuclear halo is also a characteristic (Fig. 4.13). Cancer cells are of two types, that is, (1) large polygonal cells with prominent cell membranes and pale cytoplasm (pale cells) and (2) small cells with eosinophilic granular cytoplasm (eosinophilic cells). According to the proportion of pale and eosinophilic tumor cells, chRCC can be divided into classical, eosinophilic, and mixed subtypes [28]. Oncocytoma also shows similar morphology to eosinophilic subtype of chRCC, and there is a small subset of renal tumors showing overlapping histology between oncocytoma and chRCC (hybrid oncocytic/chromophobe tumors). A diffuse cytoplasmic staining reaction with Hale’s colloidal iron stain is a diagnostic hallmark and characteristic feature (Fig. 4.14). Immunohistologically, cancer cells are positive for CK7, CD82, ERA (MOC31), parvalbumin, CD117 (c-kit), E-cadherin, and kidney-specific cadherin, and they are usually negative for CA9, CD10, AMACR, and RCC-Ma (Fig. 4.14) [18]. Strong cytoplasmic CK7 staining may be a characteristic feature of this tumor, whereas it is negative or focally positive in renal oncocytoma (Fig. 4.15). Many chRCCs show distinct and peripheral cytoplasmic accentuation of CD117 staining (Fig. 4.14), whereas oncocytoma shows cytoplasmic positivity (Fig. 4.15) [18, 29]. Generally, chRCC has a favorable prognosis and tumor state, and sarcomatoid change, necrosis, and microvessel invasion are the predictors of the patients with chRCC [1]. WHO/ISUP grading system should not be applied to chRCC, because chRCC has its innate nuclear atypia [1].
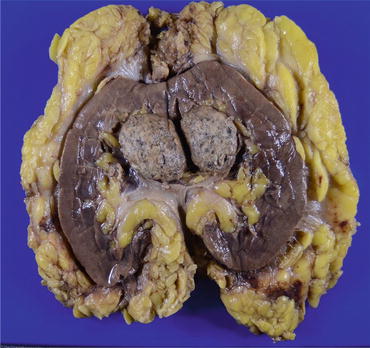
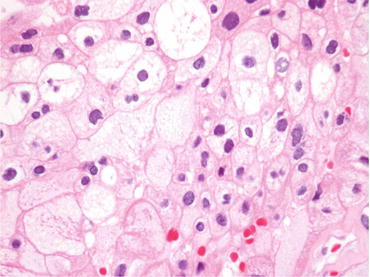
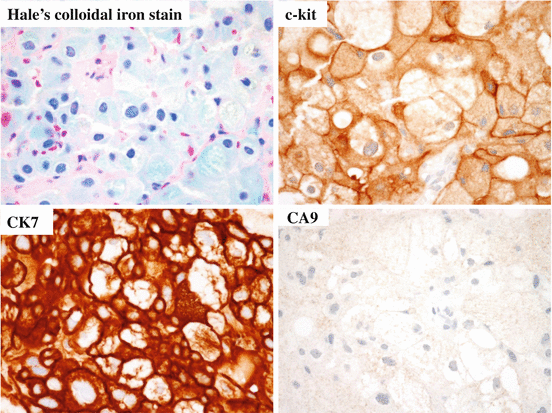
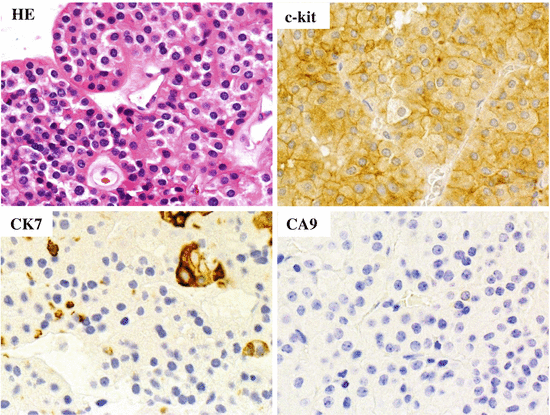

Fig. 4.12
Macroscopic appearance of chRCC. The gray tumor is solid and well circumscribed

Fig. 4.13
Microscopic appearance of chRCC. Cancer cells have a distinct cell border and perinuclear halo, and there are large polygonal cells with pale cytoplasm and small cells

Fig. 4.14
chRCC is positive for Hale’s colloidal iron stain, CD117, and CK7 and negative for CA9

Fig. 4.15
Microscopic appearance and immunostaining of oncocytoma. Microscopically, oncocytoma is composed of small eosinophilic cells, and it is weakly positive for CD117 staining and negative for CA9. Many tumor cells are negative for CK7, but a small number of tumor cells are positive
4.5 Collecting Duct Carcinoma of Bellini
4.5.1 General Features
CDC is a subtype of RCC, which is considered to be derived from the principal cells of the collecting duct of Bellini, and it accounts for about 1 % of all renal tumors [1]. The mean age of the patients is about 50 years (range, 13–83 years) with a male-to-female ratio of 2–3:1 [30]. Abdominal pain, flank mass, and hematuria are frequently observed symptoms of patients with CDC. About one-third of patients have metastases at the time of diagnosis.
4.5.2 Morphological Features
Grossly, small CDCs are predominantly located in the medulla of the kidney. However, many CDCs are advanced tumors, and they diffusely involve the renal cortex (Fig. 4.16). Their cut surfaces show a firm gray-white appearance with irregular borders [30]. The diagnosis of CDC is difficult in many cases, and an exclusive diagnosis of other histological subtypes of RCC, urothelial carcinoma, and metastatic carcinoma using immunohistochemistry is mandatory. Microscopically, CDC has a tubular to papillary growth pattern with diffuse infiltration to renal parenchyma accompanied by a desmoplastic stroma, and the boundary of the tumor is usually poorly defined (Fig. 4.17) [31]. A sarcomatoid or rhabdoid transformation, which is similar to that seen in other RCCs, has been also reported [32]. Cancer cells of CDCs usually show high-grade (Fuhrman nuclear grades 3 and 4) nuclear features with eosinophilic cytoplasm. The surrounding collecting ducts sometimes show dysplastic change.
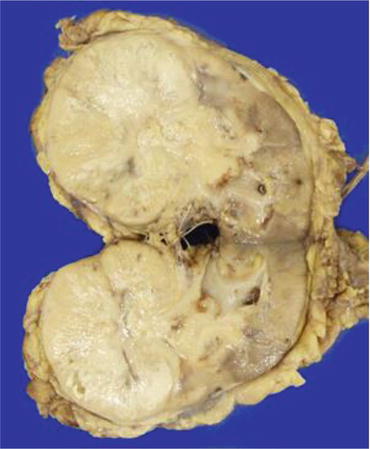
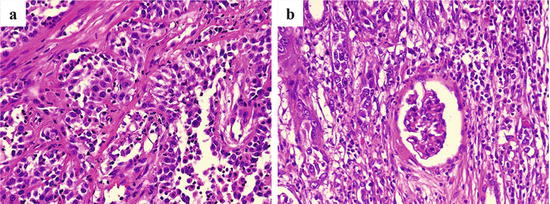

Fig. 4.16
Gross appearance of CDC. The cut surfaces of the tumor have a firm gray-white appearance with irregular borders

Fig. 4.17
Microscopic appearance of CDC. Cancer cells proliferate in a tubular to papillary growth pattern (a), and they diffusely infiltrate to nonneoplastic renal tissues with inflammation and fibrosis (b)
Immunohistochemically, tumor cells are positive for vimentin, CK7, CK19, PAX2, and PAX8 but negative for CK5/6, CK17, kidney-specific cadherin, CD10, RCC-Ma, CD117, p63, and CK20 (Fig. 4.18) [18]. Positive staining for CK (34βE12) and Ulex europaeus agglutinin 1 (UEA-1) was reported as one of the diagnostic criteria in the previous WHO classification [14], but immunohistochemistry is not included in the criteria of updated WHO classification. Recently proposed six histological diagnostic criteria are medullary involvement, predominantly tubular morphology, desmoplastic stromal reaction, cytological high grade, invasive growth pattern, and absence of renal cell carcinoma subtypes or urothelial carcinoma [1]. CDC is usually negative for the markers of ccRCC (CA9), pRCC (AMACR), and urothelial carcinoma (p63, GATA3, and uroplakins). Metastases are found in many patients at presentation, and about two-thirds of the patients die of the disease within 2 years of diagnosis [30].
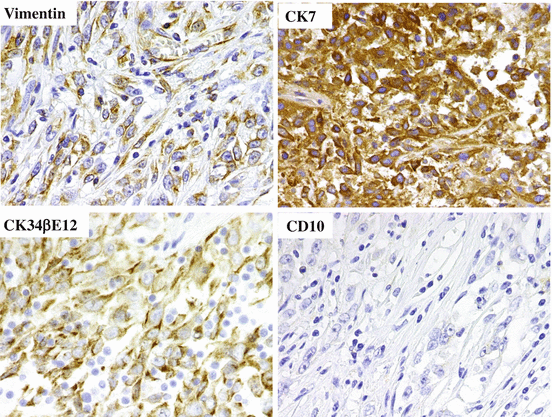

Fig. 4.18
Immunostaining of CDC. The tumor cells are positive for vimentin, CK (34βE12), and CK7 and negative for CD10
4.6 Mucinous Tubular and Spindle Cell Carcinoma (MTSCC)
4.6.1 General Features
4.6.2 Morphological Features
Grossly, MTSCCs are well-circumscribed solid tumors, and their cut surfaces are gray or light tan (Fig. 4.19). Microscopically, cuboidal tumor cells proliferate in cord-like, tubular, and papillary structures, and there are also small spindle cells with low nuclear grade (Fig. 4.20). Another characteristic feature of MTSCC is mucinous stroma, which is demonstrated by Alcian blue staining (Fig. 4.21). Cancer cells are positive for vimentin, EMA, CK (AE1/AE3), CK7, CK8, CK18, CD15, and AMACR (Fig. 4.21), and there is considerable similarity of immunostaining between MTSCC and pRCC [18]. Therefore, the differential diagnosis of MTSCC and pRCC is often challenging. Although many MTSCCs were considered as low-grade RCC at first, tumors with poor prognosis have been reported recently [33, 34]. Sarcomatoid change is also observed [35]. Although most MTSCCs have an indolent course, tumors with high-grade transformation may show distant metastasis and can be fetal [1].
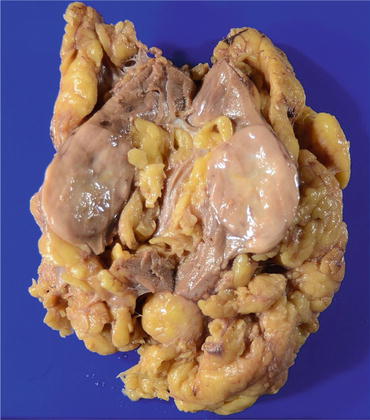
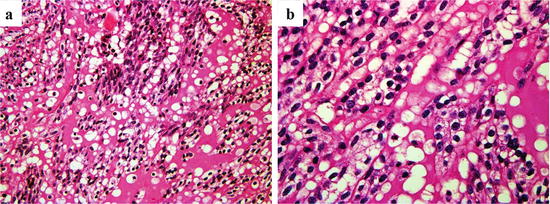
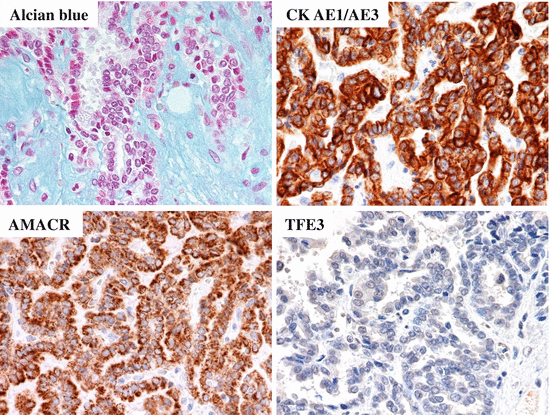

Fig. 4.19
Macroscopic appearance of MTSCC. The tumor with a light tan color is solid and well circumscribed

Fig. 4.20
Microscopic appearance of MTSCC. Cuboidal tumor cells proliferate in tubular and papillary structures (a), and there are also small spindle cells with low nuclear grade (b)

Fig. 4.21
Alcian blue staining and immunostaining of MTSCC. Alcian blue staining reveals mucinous stroma. Tumor cells are positive for AMACR and CK (AE1/AE3) and negative for TFE3
4.7 MiT Family Translocation RCCs
4.7.1 General Features
MiT family translocation RCCs are defined as renal tumors harboring gene fusions involving TFE3 or TFEB of the MiT family translocation factors [1]. The gene fusions of RCC associated with Xp11 (Xp11 RCC) include ASPCR1/ASPL-TFE3 (alveolar soft part sarcoma critical region 1/alveolar soft part sarcoma locus-TFE3), PRCC-TFE3 (papillary renal cell carcinoma-TFE3), and other minor ones such as PSF-TFE3 (PTB-associated splicing factor-TFE3), NONO-TFE3, and CLTC-TFE3. Xp11 RCCs tend to affect children and young adults, but some older patients with this tumor have also been reported [36]. RCC with t(6; 11) (6p21 RCC) also occurs predominantly in children and young adults and has a translocation between the TFEB gene located on chromosome 6q21 and the Alpha gene located on chromosome 11q12. 6p21 RCCs are less common than Xp11 RCC [1].
4.7.2 Morphological Features
No distinctive gross appearance has been known in MiT translocation RCCs. A case of Xp11 RCCs shows tan-yellow and solid tumors (Fig. 4.22). Microscopically, many Xp11 RCCs are composed of a carcinoma with papillary, alveolar, or solid architecture comprised of clear to eosinophilic cells. The ASPCR1/ASPL-TFE3 tumors have abundant clear to eosinophilic cytoplasm, discrete cell borders, vesicular chromatin, and prominent nucleoli. Psammoma bodies and hyaline nodules are often observed in the stroma [37]. The PRCC-TFE3 tumor cells have less abundant cytoplasm and fewer psammoma and hyaline nodules, and the tumor cells form more nested, compact architecture [36]. 6p21 RCC is composed of nests of large and small cells. Rosette-like structures surrounding basement membrane-like materials are an important diagnostic clue.
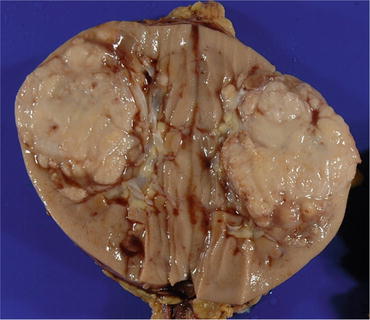

Fig. 4.22
Gross appearance of Xp11 RCC. The tumor is tan yellow and solid
Because TFE3 gene fusions lead to overexpression and retention of fusion protein compared with native TFE3, strong immunohistochemical nuclear staining for TFE3 is a sensitive and specific marker for Xp11 RCC (Fig. 4.23) [38]. However, as TFE3 is ubiquitously distributed in the normal human body, immunohistochemical factors such as excessive antigen retrieval, high antibody concentration, and excessive signal amplification could lead to false-positive results [38]. Appropriate positive (alveolar soft part sarcoma or Xp11 RCC diagnosed by genetic analysis) and negative controls should be simultaneously stained for TFE3 immunostaining. Therefore, the detection of TFE3 gene translocation by RT-PCR or FISH is necessary for the definitive diagnosis of this tumor. Only about 50 % express epithelial markers such as CK and EMA, and Xp11 RCC is positive for RCC-Ma and CD10 [36, 37]. Nuclear staining for TFEB is a highly sensitive and specific marker for 6p21 RCC, and this tumor is frequently positive for melanoma markers and usually negative for epithelial antigen markers.
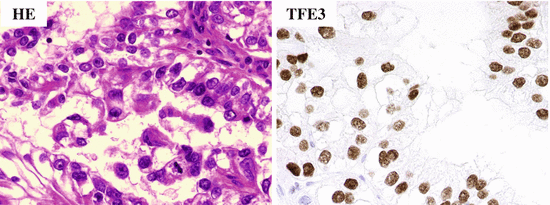

Fig. 4.23
Microscopic appearance and TFE3 immunostaining of Xp11 RCC. The tumor cells proliferate in a papillary pattern and their nuclei are positive for TFE3
4.8 Dialysis-Related RCC
Long-term hemodialysis causes atrophy and cystic change of the renal parenchyma, which is called acquired cystic disease (ACD) of the kidney. About 3–7 % of patients with ACD develop RCC [39, 40]. Although ccRCC, pRCC, and chRCC may occur, there are unique subtypes of RCC associated with long-term dialysis, such as ACD-associated RCC and clear cell papillary RCC.
4.8.1 ACD-Associated RCC
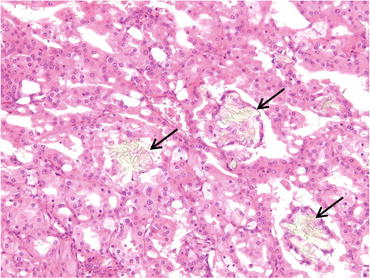
This tumor is common in the patients receiving long-term dialysis and occurs with the background of ACD. Grossly, it is well circumscribed with hemorrhage and necrosis (Fig. 4.24). Microscopically, this tumor is characterized by cribriform architecture containing numerous microcysts with eosinophilic cytoplasm, prominent nucleoli, and calcium oxalate crystals (Fig. 4.25) [1]. Immunohistochemically, cancer cells are positive for AMACR, RCC-Ma, CK (AE1/AE3), CK (CAM5.2), and CD10 but negative for cytokeratin CK7, EMA, CK (34βE12), CD117, and TFE3. This immunophenotype seems to resemble that of type 2 pRCC. This tumor generally shows a favorable prognosis, but patients having tumor with metastasis and/or sarcomatoid change show worse clinical outcomes [41].
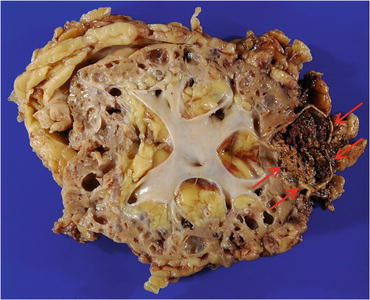

Fig. 4.24
Gross appearance of ACD-associated RCC. The tumor is well circumscribed with hemorrhage and necrosis (arrows). Nonneoplastic renal tissue is occupied by numerous cysts