Pathology of Bone Metastases— Implications for Treatment
Patrick W. Mantyh
INTRODUCTION
Similar to cancer itself, the factors that drive cancer pain evolve and change with disease progression. Surgery, radiation, and chemotherapy are used to remove or kill cancer cells and all can induce pain and/or dysfunction of sensory and sympathetic nerve fibers. In cases where the cancer continues to grow or relapse occurs, cancer cells and their associated stromal cells generate an ongoing pain by releasing algogenic substances, including protons, bradykinin, endothelins (ETs), prostaglandins, proteases, and tyrosine kinase activators. With disease progression, tumor growth can directly injure nerve fibers giving rise to a neuropathic pain. Additionally, cancer and associated stromal cells can release growth factors such as nerve growth factor (NGF) that can induce a highly ectopic sprouting and formation of neuroma-like structures by sensory and sympathetic nerve fibers.
This structural reorganization of sensory and sympathetic nerve fibers along with a cellular and neurochemical reorganization in the spinal cord and brain has the potential to drive breakthrough cancer pain. Incorporating this new understanding of the mechanisms that drive cancer pain into new treatments for cancer pain should increase the quality of life and functional status of cancer patients and survivors.
THE CLINICAL PROBLEM
In 2008, over 12 million people were diagnosed with cancer worldwide (excluding non-melanoma skin cancer) and 8 million individuals died from cancer (1). By 2030, the global burden is expected to grow to 21.4 million new cancer cases and 13.2 million cancer deaths simply due to the growth and aging of the population, as well as reductions in childhood mortality and deaths from infectious diseases in developing countries. Cancer incidence rates are stable or slightly falling in developed countries, whereas in developing countries cancer incidence rates are increasing, as smoking, obesity, and increased life expectancy have led to a rapid rise in the incidence of cancer (1,2,3). Additionally, as the detection and treatment of most cancers have dramatically improved, survival rates have increased so that even patients with metastatic cancer are surviving years to decades beyond their initial diagnosis (4).
Cancer-associated pain is one of the most common presenting symptoms that results in the diagnosis of cancer. While pain can be present at any time during the course of the disease, it generally increases with disease progression so that 75% to 90% of patients with metastatic or advanced stage cancer will experience significant cancer pain (5,6). Mechanisms that drive cancer pain include cancer therapy, factors released from tumors that sensitize or excite primary afferent neurons, tumor-induced nerve injury, and tumorinduced nerve sprouting and neuroma formation (7,8,9,10).
In patients with cancer, having surgery or receiving the full dose of radiation or chemotherapy is one of the most significant factors in determining patient survival (7). However, peripheral nerve neurotoxicity and accompanying pain are major side effects of radiation and many of the most commonly used anti-neoplastic agents, including the taxanes (e.g., paclitaxel and docetaxel), vinca alkaloids (e.g., vincristine and vinblastine), platinum-based compounds (e.g., cisplatin and oxaliplatin), and proteasome inhibitors (e.g., bortezomib and disulfiram) (7,11,12,13,14). If the chemotherapy-induced peripheral neuropathy becomes severe enough, the oncologist or patient may reduce or cease chemotherapy treatment, which decreases the overall survival rate of the patient and the likelihood that the patient will be disease free.
In cases where the tumor is inoperable or relapse occurs, cancer and associated stromal cells can induce significant pain. This pain can arise from the original site of the cancer (i.e., pancreatic cancer, head and neck cancer, and osteosarcoma) (15,16,17) or from distant sites (such as bone) where common cancers such as breast, prostate, kidney, and lung avidly metastasize (18). The original presentation of tumorinduced pain is usually described as dull in character, constant in presentation, and gradually increasing in intensity with time (19). If the disease progression continues, a second type of cancer pain known as “breakthrough” or severe “incident pain” can emerge (20). Incident or breakthrough pain, which is defined as a transitory flare of extreme pain superimposed on an otherwise stable pain pattern in patients treated with opioids (21), can occur spontaneously or by the movement or weight bearing of a tumor-bearing organ or tissue (22). Since breakthrough pain is frequently acute and unpredictable in onset, this pain can be severe, debilitating, and difficult to fully control (23,20).
Currently, tumor-induced pain is largely managed with an “analgesic ladder” that was originally promulgated by the World Health Organization in 1986. This ladder begins with a non-steroidal anti-inflammatory drug (NSAID) and if pain worsens the next step is NSAID + a mild opiate and finally when the pain becomes severe, an NSAID + a strong opiate.
In addition to this three-step ladder, other adjuvant therapies including radiation therapy, radioisotopes, nerve block, nerve lesions, antiepileptics (e.g., gabapentin and carbamazepine), antidepressants (e.g., amitriptyline and imipramine), and steroids are commonly employed to control cancer pain (24). It should be stressed that most cancer pain can be controlled if the cancer pain is closely monitored and these therapies are used in a timely and proactive manner. However, all of the above therapies have significant unwanted side effects (25) and closely monitoring and fully controlling cancer pain (especially if breakthrough pain is present) can be very time consuming for the patient, caregiver, and physician (26). Developing new analgesic therapies that are more efficacious and have fewer side effects than current analgesics and incorporating these advances into mainstream cancer therapy will significantly improve the quality of life and functional status of both the patient and the caregiver.
In addition to this three-step ladder, other adjuvant therapies including radiation therapy, radioisotopes, nerve block, nerve lesions, antiepileptics (e.g., gabapentin and carbamazepine), antidepressants (e.g., amitriptyline and imipramine), and steroids are commonly employed to control cancer pain (24). It should be stressed that most cancer pain can be controlled if the cancer pain is closely monitored and these therapies are used in a timely and proactive manner. However, all of the above therapies have significant unwanted side effects (25) and closely monitoring and fully controlling cancer pain (especially if breakthrough pain is present) can be very time consuming for the patient, caregiver, and physician (26). Developing new analgesic therapies that are more efficacious and have fewer side effects than current analgesics and incorporating these advances into mainstream cancer therapy will significantly improve the quality of life and functional status of both the patient and the caregiver.
THE TUMOR ENVIRONMENT AND CANCER PAIN
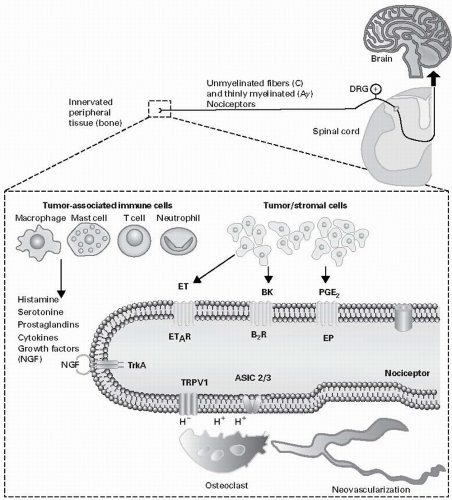
The tumor is composed of not only cancer cells but also tumorassociated stromal cells. In most tumors, stromal cells far outnumber the cancer cells and include endothelial cells, fibroblasts as well as a host of inflammatory and immune cells, including macrophages, mast cells, neutrophils and T lymphocytes (27). Both cancer cells and their associated stromal cells secrete a wide variety of factors (7,27), many of which have been shown to sensitize or directly excite primary afferent neurons (28).
Tumor-Induced Acidosis
The finding that a subpopulation of sensory neurons (Figure 5.1) expressed the transient receptor potential vanilloid 1 (TRPV1, which is also known as the capsaicin receptor) and the acid-sensing ion channel 3 (ASIC3) and that both these channels responded to acidosis was of significant interest to researchers studying cancer pain (28). Thus, cancer cells in general have a lower pH (6.8) than normal cells (pH 7.2) (29). Importantly, many tumors that metastasize to bone induce a marked proliferation and hypertrophy of osteoclasts. Osteoclasts avidly resorb bone by generating a pH of 2 to 4 in their resorption bay, which contributes to the excessive bone resorption that can ultimately lead to fracture of the tumor-bearing bone (30). To test whether TRPV1 channels are expressed by sensory nerve fibers that innervate tumor-bearing tissue and whether a TRPV1 contributed to cancer pain, an in vivo mouse model of bone cancer pain was explored (31). In these studies it was shown that a subpopulation of nerve fibers that innervate the tumor-bearing bone expressed TRPV1, that acute or chronic administration of a TRPV1 antagonist attenuated bone cancer pain, and that disruption of the TRPV1 gene results in an attenuation of bone cancer pain (31). Furthermore, administration of the TRPV1 antagonist to TRPV1 null animals with bone cancer resulted in no further reduction of pain than what was already present in the TRPV1 null mice (31). To date results from human clinical trials with TRPV1 or ASIC3 channel antagonists have not been reported in cancer pain. However, as discussed below, understanding the role that TRPV1 plays in driving bone cancer pain has provided insight into one mechanism by which therapies that inhibit osteoclasts (including bisphosphonates and denosumab, which is an anti-RANKL [anti-receptor activator of nuclear factor kappa-B ligand] fully humanized monoclonal antibody) are efficacious in reducing bone cancer pain (32,33,34).
The skeleton is the most common site for distant metastasis of prostate, breast, lung, and renal carcinomas (18). Once tumor cells have metastasized to bone, a cycle of tumor growth, bone destruction, and formation of woven bone begins, which can result in significant pain, skeletal fractures, and hypercalcemia (18). Cancer cells themselves do not destroy bone but rather they and their associated stromal cells express the RANKL which binds to its receptor RANK that is expressed by osteoclasts. The activation of the RANKL/RANK pathway promotes the proliferation and hypertrophy of these bone-destroying osteoclasts (35). Osteoclasts resorb bone by forming a highly acidic resorption “bay” or “pit” between the osteoclast and bone, which can stimulate the TRPV1 or ASIC3 channels and drive bone cancer pain (35). In the last decade, multiple studies have shown that therapies that reduce osteoclast function also significantly reduce bone cancer pain (7,32,33,34,36,37).
The first and most widely used therapy is the class of compounds known as bisphosphonates that avidly bind to bone. Once the bisphosphonate has bound to bone, osteoclasts that are resorbing bone generally need to actively endocytose the breakdown products of bone at the apical (bone facing) surface and transcytose these products to be released at the distal surface of the osteoclast for disposal by exocytosis (38). However, if a bisphosphonate is tightly bound to the bone that is being resorbed, the bisphosphonate will also be taken up by endocytosis (39). Once internalized, the bisphosphonate interferes either with adenosine triphosphate energy metabolism (nonnitrogen-containing bisphosphonates) or with the mevalonate pathway (nitrogen-containing bisphosphonates), resulting first in osteoclast dysfunction and ultimately in osteoclast apoptosis (39,40). As a significant population of nerve fibers that innervate the bone express TRPV1 (31), one way bisphosphonates appear to relieve bone pain is by decreasing the osteoclast-induced acidosis, which in turn decreases the activation of the ion-sensing TRPV1 or ASIC3 receptors that are expressed by sensory nerve fibers.
Another method that is highly effective in reducing tumor-induced osteoclast bone resorption in both animals and humans is by interfering with the RANKL binding to RANK, which is required for osteoclast proliferation, maturation, and survival (41). Within 2 days of administration of therapies that interfere with RANKL binding to RANK (such as osteoprotegerin or denosumab), there is almost a complete loss of activated osteoclasts, a marked reduction in plasma markers of bone resorption, and a significant attenuation of bone cancer pain (36).
Tumor-Induced Mechanical Instability of Bone
Therapies that inhibit osteoclast-induced bone resorption also maintain the mechanical strength of bone even though tumor cells are present in the bone. Thus, in addition to acidosis, excessive tumor-induced osteoclast bone resorption destroys bone and leads to mechanical instability and fracture of bone that causes mechanical distortion of the sensory nerve fibers that innervate the bone (42,43). Thus, following significant weakening or fracture due to tumor-induced bone remodeling, there can be significant movement-evoked pain presumably due to mechanical distortion of the mechanosensitive sensory nerve fibers that innervate the bone. Clearly, pain associated with fracture and distortion of the bone is usually attenuated if the bone is stabilized and repositioned into its normal orientation (44). Both osteolytic and osteoblastic tumors induce a loss of the mechanical strength and stability of mineralized bone (45,46), so with significant bone remodeling, bone stress that is normally nontraumatic can now result in bone fracture with resulting distortion and activation of mechanosensitive nerve fibers that innervate the bone. As bisphosphonates and anti-RANKL therapies reduce tumor-induced osteoclast bone remodeling, preserve the mechanical strength of bone, reduce bone fracture, and reduce osteoclast-induced acidosis in both animals and humans, these therapies are now routinely used to prevent cancer-induced bone fractures and manage pain due to cancer metastasis to bone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree