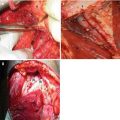
Fig. 4.1
Penile cancer. Exophytic lesion
Stage is important in the treatment of primary lesion. In a study with 196 patients, a local extension of the primary tumor into corpora cavernosa was found in 44.9 % of patients. The corpus spongiosum and urethra were involved in 21.4 and 35.2 % of cases, respectively [1].
Pathological factors with a known prognostic value, other than lymph node metastasis, are tumor thickness, grade, histological type, lymphovascular embolization, and stage [2, 3].
The rate of depth invasion is significantly high when the thickness is more than 5 mm. The histological grade of penile carcinoma, including SCCP usual type, should attend the protocol based on the American Joint Committee on Cancer, TNM, 7th edition [4]:
(G1) Well-differentiated carcinoma, tumors with a minimal deviation from the morphology of normal/hyperplastic squamous epithelium (Fig. 4.2)
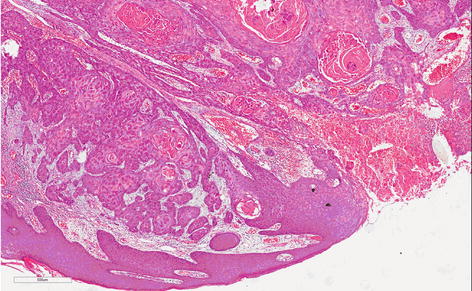
Fig. 4.2
Usual grade 1, well-differentiated SCC. Proliferation of mature epithelial cells with basal atypia forming nets. Abundant centralized keratin pearl
(G2) Moderately differentiated carcinoma constitutes the great part of the cases; tumors show a more disorganized growth as compared to grade 1 lesions, increased nucleus-cytoplasm ratio, evident mitoses, and, although present, less prominent keratinization (Fig. 4.3)
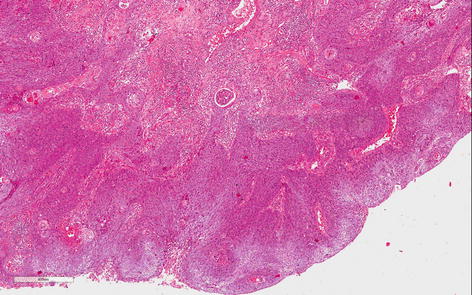
Fig. 4.3
Usual grade 2, moderately differentiated SCC. Less keratin and cell differentiation than grade 1. Irregular infiltrative borders, nuclear atypia, evident mitosis
(G3) Poorly differentiated carcinomas are tumors showing any proportion of anaplastic cells, identified as solid sheets or irregular small aggregates, cords, or nests of cells with little or no keratinization, high nucleus-cytoplasm ratio, thick nuclear membranes, nuclear pleomorphism, clumped chromatin, prominent nucleoli, and numerous mitoses (Fig. 4.4)
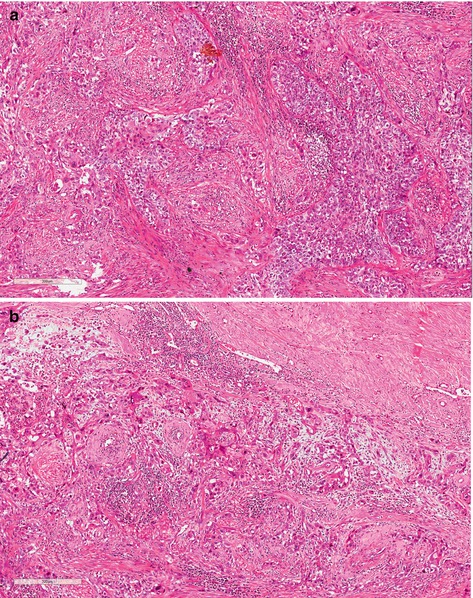
Fig. 4.4
(a) Usual grade 3, poorly differentiated SCC. Prominent stromal desmoplastic tissue involved solids nets and irregular small aggregates of anaplastic cells. Individual keratinized cells are present. (b) Usual grade 3, poorly differentiated SCC. Sheets, cords, and small aggregates of anaplastic squamous cells, isolated keratinized cells, nuclear pleomorphism, hyperchromatin, prominent nucleoli, and numerous mitoses
A tumor should be graded according to the least differentiated component. Any proportion of grade 3 should be distinguished in the description. Patients with well-differentiated carcinoma have a higher 10-year survival rate than those with moderately and poorly differentiated carcinoma (P < 0.0001 and P = 0.006) [5].
Lymphovascular embolization and absent koilocytosis have proved to be independent prognostic factors for the risk of lymphatic metastasis. Patients with koilocytosis and without lymphovascular embolization had better 5-year survival [1].
SCCP can be seen as usual SCCP type or constituting the variant forms or subtypes sometimes associated with HPV. Basically the grossly usual SCCP shows itself either as a flat, an endophytic, or as an exophytic tumor (cauliflower appearance), white-gray and firm tumor with necrosis foci. Microscopically it consists of squamous cell proliferation that may infiltrate superficially or profoundly penile tissues and/or projecting outside itself with variable degrees of cytological atypia, mitotic figures, and keratin that can be seen as mild or prominent keratinized cell [6, 7].
Variations of squamous cell carcinoma or subtypes can be seen forming two groups: high-grade and low-grade groups. The high-grade tumors (basaloid and sarcomatoid) frequently are associated with deeper invasion, recurrence of tumors, lymph node metastasis, and significant mortality rate [8]. Low-grade variants (papillary, warty, and verrucous) have mild to moderate morbidity features and better survive rate [9]. The following are a short description of them:
Basaloid carcinoma (BC) is a high-grade subtype associated to HPV and can be seen as flat, endophytic, or papillar [10]. Macroscopic features are ulcerated lesion, flat or slightly elevated, and firm. Microscopy is infiltrated tumor made of cell nests with few cytoplasm and basophilic nuclei, centered by comedonecrosis. Nucleus is oval or round, pleomorphic and hyperchromatic, with inconspicuous nucleoli and numerous mitoses (Fig. 4.5).
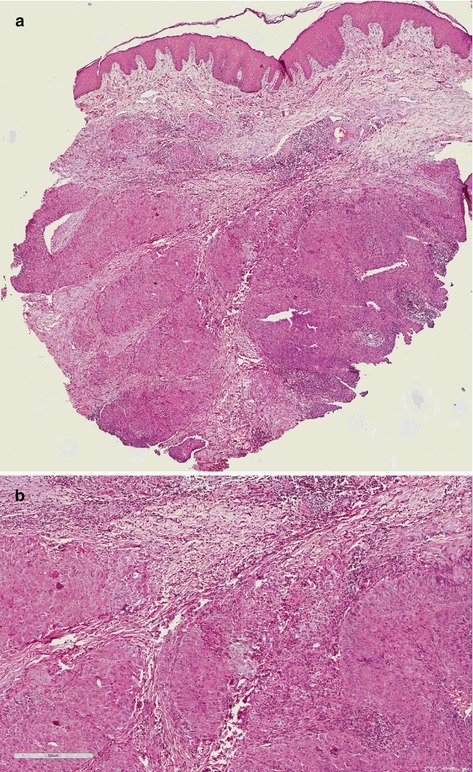
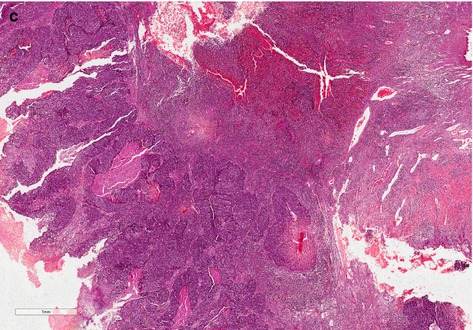


Fig. 4.5
(a) High-grade SCC, basaloid subtype (panoramic vision). Endophytic infiltrative tumor of basaloid cells. (b) High-grade SCC, basaloid variant group. Note irregularity of the tumor nets with ragged basal membrane showing its aggressiveness. Cells present poor cytoplasm, great oval or round basophilic nuclei with prominent nucleolus, and atypical mitoses. (c) Basaloid SCC subtype. Tumor shows papillary appearance. Comedonecrosis involved by basaloid cells
Sarcomatoid carcinoma (SC) is a primary spindle-cell squamous carcinoma, rare in penis, similar to a sarcomatous tumor, aggressive with deep structural infiltration, poor prognosis, associated with lymphatic, and hematogenous spread (Fig. 4.6). Metastatic disease develops in a high percentage of cases. Distant tumor metastases occur mainly in lung, skin, bone, and pericardium and pleura [11, 12].
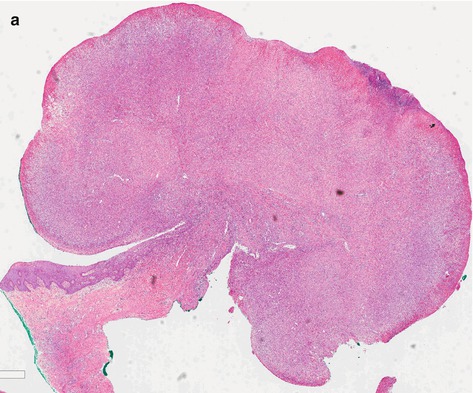
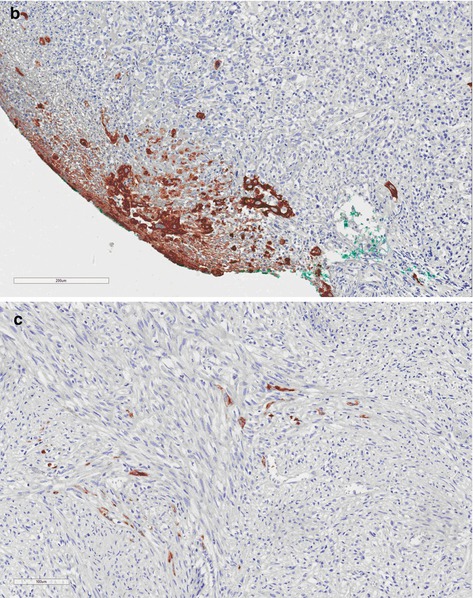


Fig. 4.6
(a) Spindle-cell carcinoma, high-grade group. Ulcerated tumor presents fusiform cells similar to those present in other sarcomatous tumors. (b and c) Sarcomatoid SCC. Groups of squamous and isolated brown cells were stained by 34BE12 keratin
Warty carcinoma (WC) is a morphologically distinct verruciformis tumor with features of HPV-related lesions [13]. HPV attacks the squamous epithelium and produces nuclear atypia, binucleation, and koilocytosis (identified by a large halo around cellular nuclei) (Fig. 4.7).
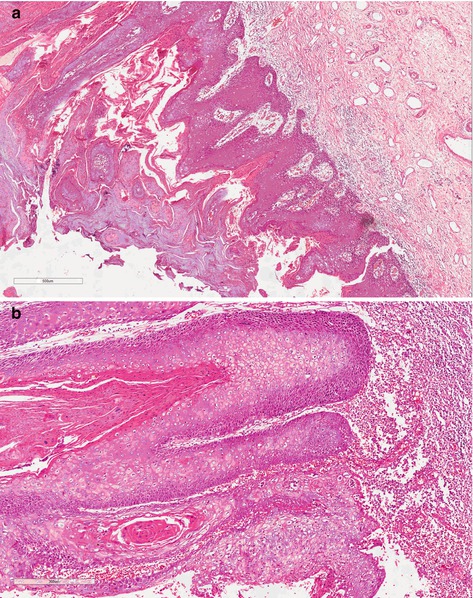

Fig. 4.7
(a) Warty carcinoma, low-grade variant. The keratin fills up the space between acanthotics cells columns with long and large papillae. (b) HPV-related warty carcinoma exhibiting darker band of basaloid tumor cells deep in the tumor and present area with clear cells and koilocytosis
Papillary carcinoma (PC) is a papillary well-differentiated carcinoma which can invade stromal tissue until deep structures but with rare lymph node involvement. The prognosis is good and tumor is associated with lichen sclerosus [9]. Grossly it is an exophytic white-gray and firm tumor. Microscopy is complex and simple papillae with fibrovascular core and hyperkeratosis.
Verrucous carcinoma (VC) is generally a large lesion (average 4 cm) with exophytic papillary growth, frequently softy, ulcerated sometimes purulent, and foul smelling tumor. Tumor can be seen anywhere on the penis, frequently on the glans and foreskin. A specific etiologic factor is not described. Microscopically, the presence of well-differentiated characteristics frequently makes hard differential diagnostics, mainly with condyloma accuminatum and well-differentiated SCC. Superficially, hyperkeratosis, acanthosis, and papillomatosis are seen, and it excavates through the normal tissue and slowly invades continuous structures (Fig. 4.8). Regional lymph node metastases are rare and distant metastases have not been reported [14].
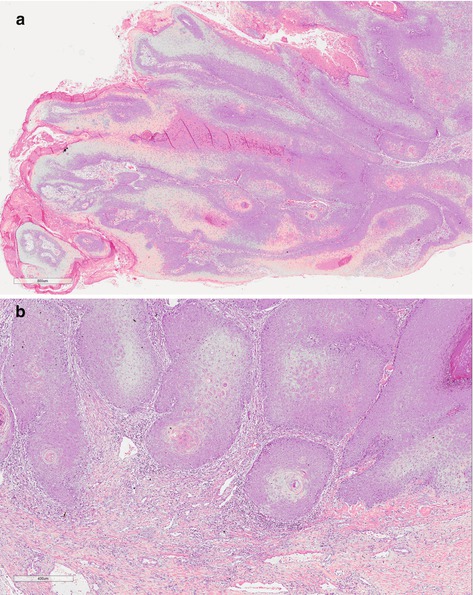

Fig. 4.8
(a) Verrucous carcinoma, low-grade variant. Tumor not associated with HPV represented by well-differentiated squamous cells with thin and long papillae containing fibrovascular cores and hyperkeratosis. (b) Verrucous carcinoma, low-grade variant. The round tumor board of squamous cell is present in stromal tissue configuring nest with single basal cells involved by inflammatory infiltrated. Inside keratinized material
Genetics
Introduction
Cancer is a disease caused by our own genes’ deregulation. Cancer cells divide indefinitely as a consequence of a deep genetic expression turning. This occurs step by step or mutation by mutation until the cell reaches the point of no return, ignoring the cell cycle checkpoints. Environmental risk factors causing mutagenesis and the failure of the mechanisms of genome repair combine to promote mutations in the key genes that control the cell cycle progress.
The key genes in the carcinogenesis process are the proto-oncogenes and the tumor suppressor genes. In normal cells, proto-oncogenes are responsible for promoting the cell growth. When altered or mutated, they become oncogenes and then can contribute to indefinitely cell division. The tumor suppressor genes that are normally present in our cells can lose their capacity of controlling the processes of cell growth and cell death (called apoptosis) when they are mutated or deleted.
Damage in the key genes that control the cell cycle is caused by many factors. Carcinogens are a class of substances that are directly responsible for promoting cancer. Tobacco, asbestos, arsenic, radiation such as gamma and x-rays, the sun (UV rays), and compounds in car exhaust fumes are all examples of carcinogens [15–18]. When our bodies are exposed to carcinogens, free radicals are formed that try to steal electrons from other molecules in the body. These free radicals damage cells and affect their ability to function normally.
Apart from the carcinogens, oncoviruses such as human papillomavirus (HPV) and Epstein-Barr virus (EBV) can help to cause penile cancers [19, 20]. The mechanism by which HPV promotes cancer is not affecting the genes. HPV encodes for E6 and E7 proteins that are able to bind to two important tumor suppressor proteins, p53 and pRB, respectively, inactivating them [21]. EBV is widely distributed in human population; however, the exact role of EBV in the development of penile cancer is not understood. A possible cooperation between EBV and HPV in the process of penile carcinogenesis should be considered [20].
Cytogenetics and Flow Cytometry Findings
Genes made of DNA are organized in a complex nuclear structure called chromosomes. A normal human cell contains 46 chromosomes. In cancer cells, chromosome rearrangements and the alteration of chromosome number can be detected with the application of cytogenetic techniques. Chromosomes in metaphase can be fixed on slides and can be observed under a microscope. The karyotype is the picture of the chromosomes from one metaphase that is arranged according to the standard classification.
In contrast to most tumors, publications about karyotype alterations in squamous cell carcinoma of the penis (SSCP) are uncommon. By conventional cytogenetics only four karyotypes were described for penile carcinoma. The rarity of karyotype description is due to technical difficulties related to the low mitotic index, contamination of primary cultures, and the occurrence of large areas of necrosis in the tumor.
The first SSCP karyotype description [22] was from a Chinese patient showing moderately differentiated SSCP (stage II). Several inguinal lymph nodes were palpable. Thirty metaphases showed the stemline karyotype 46,XY,del(2)(q33q36),der(4)t(4;?)(p16;?),der(5;15)(q10;q10),der(8)t(8;?13)(q21;?),-13,- 13,-15,+3mar. Twelve cells had the stemline pattern with additional chromosome aberrations. Chromosomes 17, 22, and Y were usually lost, whereas chromosome 14 was frequently trisomic. Five to eight markers were observed in each cell of this population. Eight polyploid cells with poor chromosome spreading were also observed. The other three described karyotypes were from Brazilian cases. The next and second karyotype description [23] was obtained from an SSCP patient who presents an advanced poorly differentiated invasive carcinoma. Cytogenetic analysis of 11 cells obtained from fresh biopsy revealed a complex karyotype nearly tetraploid: 88, XY, + der. X, t (X; ?), (q28; ?), + del. (1), (p36), + 1, + 1, + 1, + 2, + 2, + 3, + 3, + 4, + 5, + 5, + 5, + 9, + 9, + 10, + 10, + 11, + 11, + 12, + 12, + 13, + 14, + 14, + 15, + 15, + 15, + der. 16, t (1; 16), (q24; p12), + 16, + 17, + 17, + 17, + 18, + 18, + 19, + 19, + 20, + 20, + 21, + 21, + mar 2, + mar 2 (Fig. 4.9). Polyploidy was confirmed by flow cytometry (Fig. 4.10). Besides the polyploidy, translocation involving chromosomes 1 and 16 and the appearance of additional genetic material on the X chromosome and isochromosome of the short arm of chromosome 10 were remarkable.
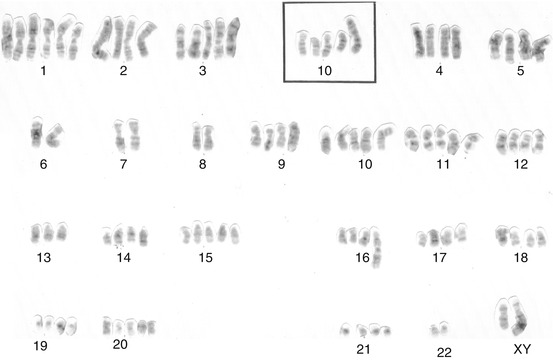
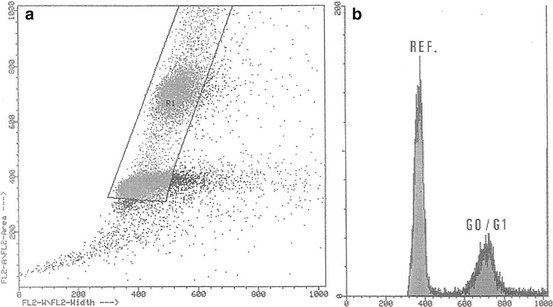

Fig. 4.9
Complete karyotype from the tumoral cells of a patient with poorly differentiated penile carcinoma, including a partial karyotype showing an isochromosome i (10) (q10) (From Ornellas et al. [23])

Fig. 4.10
Distribution pattern of the nuclear DNA content (near-tetraploid tumor). (a) Gated events displayed in identified singlet nucleus populations. (b) Singlet nucleus fluorescence intensity showing a near-tetraploid population and the diploid reference cells (From Ornellas et al. [23])
In contrast, the karyotypes of two early-stage SSCP patients showed no polyploidy [24]. One patient, with clinical stage T2N2MX, presented a moderately differentiated carcinoma of the penis, with a DNA pattern diploid. Cytogenetic analysis of 43 cells showed no cytogenetic alteration. The other patient, with clinical stage T3N1MX, presented also a moderately differentiated penile carcinoma. Cytogenetic analysis of 11 cells showed an altered karyotype (49,XY, dup(1)(q21q32), i(1)(q10), -3, add(11)(q23), del (12)(p12),+i(18)(q10), +3 mar) with a DNA pattern hyperdiploid (Figs. 4.11 and 4.12). Although few, these karyotype descriptions suggest the possibility of the patients’ present different tumors with different behaviors and that polyploidy could be a characteristic of advanced SSCP. Patient with normal karyotype had probably submicroscopic changes responsible for tumor development. In the future, a combination of cytogenetic, molecular, and histopathological stage and grade analyses will contribute to decisions on the classification of a particular solid tumor, leading to better predictive and prognostic information.
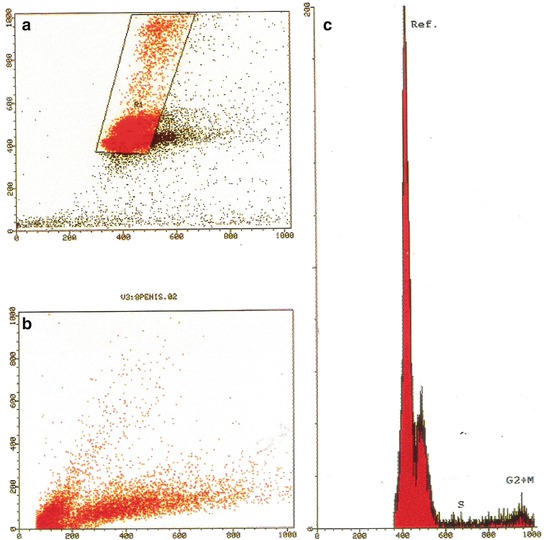
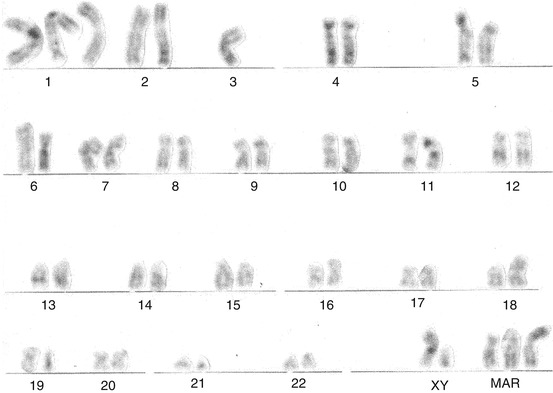

Fig. 4.11
Distribution pattern of nuclear DNA content (aneuploid tumor). (a, b) Hyperdiploid cell line was identified. (c) Singlet nuclear fluorescence intensity showing a hyperdiploid population with DNA index of 1.15 and the diploid reference cells (c, From Ornellas et al. [24])

Fig. 4.12
Hyperdiploid moderately differentiated squamous cell carcinoma of the penis with chromosomal alterations. G-banded karyotype showed 49,XY, dup(1)(q21q32), i(1)(q10), 23,add(11)(q23), del(12)(p12),1i(18)(q10),13mar. Primary culture revealed 11 metaphases (From Ornellas et al. [24])
Flow cytometry is a technique for counting and examining microscopic particles, such as cells and chromosomes, by suspending them in a stream of fluid and passing them by an electronic detection apparatus. A chromosome counting performed by flow cytometry [25] from 90 SSCP patients showed that ploidy in these tumors is proportional to the degree of cellular differentiation. The frequency of DNA aneuploidy showed correlation with histological type of invasive squamous cell carcinoma of the penis. Preliminary analysis of these cases suggested that patients with high DNA index may be at increased risk of metastatic involvement and highlights that aneuploidy seems to be a risk factor for metastatic dissemination [25].
An original molecular cytogenetics study of penile carcinoma [26] was performed by comparative genomic hybridization (CGH). The CGH technique has a great advantage over conventional cytogenetics since it is not necessary to make the primary culture of tumor cells for obtaining slides with chromosome preparations. Only DNA extraction from the tissues is necessary. This technique generates a genomic map that leads to the discovery of chromosomal regions greatly amplified in tumors as well as regions in which there were large deletions. The procedure requires different fluorochromes for labeling of DNA that was extracted from a tumor (tumor DNA), and DNA was extracted from one tissue tumor-free (normal DNA). The tumor and normal DNAs compete for hybridization in normal chromosomes, and regions with large chromosomal amplifications or deletions in chromosomes are distinguished by a computer program associated with a fluorescence microscope according to the difference of intensity of labeled tumor and normal DNA which hybridized to normal chromosomes.
DNA samples from 26 SSCP cases were assayed by CGH; six tumors were well-differentiated invasive tumors and the other 20 tumors moderately differentiated. The changes observed were similar to those described in other squamous cell carcinomas, such as oral and nasopharynx. This finding indicates that epidermoid tumors of various organs can be originated from similar genetic alterations. The regions of gene amplification observed most common were 8q24, 16p11-12, 20q11-13, 22q, 19q13, and 5p15, and the regions with the most frequent deletions were 13q21-22, 4q21-23, and along the X chromosome. In the region 8q24, the proto-oncogene MYC that encodes for transcription factor showing a helix-loop-helix leucine zipper protein domain has been mapped. The friendly MYC regulates expression of numerous target genes that control key cellular functions, including cell growth and cell cycle progression. MYC also has a critical role in DNA replication. Any kind of deregulation on MYC leads to constitutive MYC activity promotes carcinogenesis.
In 79 cases of SSCP including 11 in situ and 68 invasive carcinomas, MYC numerical aberrations and c-MYC protein expression were determined and correlated with the clinicopathological parameters and the HPV infection status of the patients [27]. The MYC cytogenetic profile was evaluated by fluorescence in situ hybridization (FISH) and c-MYC protein by immunohistochemistry (IHC). HPV was detected by polymerase chain reaction amplification (PCR). MYC gains were found to increase gradually as penile squamous cell carcinoma progresses from in situ to invasive. A significant association between MYC gains and tumor progression and poor outcome was demonstrated (p < 0.05). These findings were independent of HPV infection. Protein c-MYC expression was increased in samples with HPV infection, probably reflecting direct activation of MYC.
Gain in the region 5p15 appears very interesting, because the gene hTERT was mapped on this region. This gene codes for the major protein of the catalytic site of telomerase, the enzyme that stabilizes the telomeres of chromosomes. The stabilization of the end of the chromosomes (telomeres) is critical for promoting immortality. This result can be correlated with the study of analysis of telomerase activity in 51 samples of penile cancer [28]. In addition, gain in region 5p15 was also found in 3/7 men with cutaneous squamous cell carcinoma that were studied by array CGH [29], suggesting that this genetic event could also occur within this histological type.
Tissue and Molecular Alterations
TP53 Tumor Suppressor Gene (p53 Protein)
In an editorial of “Molecule of the Year,” p53 was described as: “The molecule p53 is a good guy when it is functioning correctly” [30]. This interpretation suggests that the normal p53 plays a role in controlling cell growth, and when mutated, the surveillance capability of the protein is eliminated and cancer can grow.
When discovered, [31] p53 was just a cellular partner bond to the simian virus 40 large T antigen. After the cloning of the gene TP53, p53 was dismissed as an oncogenic protein and recognized as a tumor suppressor that is very frequently mutated in human cancer. Since then more functions for p53 have been revealed [32]. It acts as a transcription factor induced by stress/DNA damage, promoting cell cycle arrest, apoptosis, and senescence, and also regulates cytokines that are required for embryo implantation and metabolic pathways. In addition, p53 promotes oxidative phosphorylation and dampens glycolysis in cells [33] and has a key role in regulating cell growth and autophagy, thereby helping to coordinate the cell’s response to nutrient starvation. An altered metabolism can contribute to malignant transformation as cancer cells [34].
Disruption of p53 function is a very common genetic event in many cancers, and there are many ways to cause it. The most common type of mutation causing inactivation of p53 is the missense mutation in the binding domain [35]. However, the SCCP might face another reality due to the common HPV infection [19, 20]. HPV encodes for E6 and E7 proteins that bind to p53 and pRb, respectively, leading to uncontrolled cell division and reduced apoptosis. In this situation, in spite of gene TP53 being intact, p53 is blocked by E6.
One study [36] with Chinese patients analyzed p53 accumulation by immunohistochemistry in a series of 42 primary penile carcinomas (seven verrucous carcinomas, 14 well-differentiated, 15 moderately differentiated, and six poorly differentiated squamous cell carcinomas) using the p53 protein-specific mouse monoclonal antibody on paraffin sections. The mutant p53 protein frequently accumulates within the cell and can be viewed on fixed tissues by immunohistochemistry with the application of specific antibodies. However, the determination of specific mutations must be detected by DNA sequencing. The p53 protein was detected in 40 % (17 cases) of the tumors. The p53 staining was not observed in six cases of penile warts nor in seven cases of verrucous carcinomas. Positive p53 staining was identified only in the less differentiated tumor cells in the periphery of the tumor cell nests in all the cases. The noninvasive dysplastic epithelium next to the tumors could also be positive for p53 protein. Furthermore, 100 % of the human papillomavirus (HPV)-positive cases showed positive p53 staining. The authors concluded that p53 accumulation is present in penile squamous cell carcinomas and adjacent noninvasive tumor cells. In agreement, another immunohistochemistry study identified the accumulation of p53 in two cases of HPV-positive (6 or 11) invasive carcinoma [37]. One of the cases carried a mutation in TP53 revealed by DNA sequencing. In contrast, another study did not detect the accumulation of p53 in 25 cases of carcinoma in situ [38]. Therefore, according to these reports, it seems that mutant p53 could play a role in the progression of malignancy into invasion, but this remains controversial. Biopsies of penile lesions were obtained for diagnostic purposes from 13 with 1 to 3 therapy-resistant genital warts or intraepithelial neoplasias. In addition, 4 archival specimens of SSCP were obtained. In the specimens, presence of HPV DNA was assayed by in situ hybridization and PCR analysis, and p53 accumulation was determined by immunohistochemistry. At the molecular level mutations in the DNA binding domain (exons 4–8) of TP53 were assayed in gel by single-strand conformation polymorphism (SSCP) after amplification by polymerase chain reaction (PCR). Band shifts were sequenced to detect possible mutations [39]. No correlation between p53 accumulation and HPV status was found. No mutations in the binding domain of TP53 were found in any of the lesions. The authors concluded that accumulation of p53 did not indicate existence of TP53 mutation in male genital warts, premalignant lesions, or malignant squamous cell carcinomas.
The accumulation of p53 and p21 (another tumor suppressor protein) in 49 SSCP cases was investigated by immunohistochemistry [40]. The accumulation of p21 and p53 was noted in 40 and 89 %, respectively, of the 47 patients with primary penile carcinoma with squamous differentiation. Positive p21 and p53 expression was also seen in two cases of Paget disease. Staining for p21 was often weak and was found in the suprabasal region of carcinomas with squamous differentiation, while p53 expression was seen in the basal region of squamous cell carcinomas. Preinvasive lesions also showed p21 and p53 expression. An inverse correlation between p53 and p21 expression (p53(+)/p21(−) or p53(−)/p21(+)) was noted in half of the squamous cell carcinomas, 4 of 5 verrucous carcinomas, 2 of 3 basaloid squamous cell carcinomas, and 1 spindle-cell carcinoma. The other cases did not show this correlation. Therefore, p21 and p53 expression seems to be independent in SCCP. The relationship between expression-positive/expression-negative p53 and p21 did not show a direct correlation with histological subtypes and staging. However, a strong association between positivity for HPV16 and p21 was found.
Alterations in p53 protein were also identified in 22 of 63 (35 %) patients with epidermoid tumors and invasive penile stage pT2-4 N1-3 MX [41]. The presence or absence of p53 in the nucleus has been correlated in primary lesions and nodal metastasis in 20 patients. Of these 20 patients (90 %), 18 agreed on the levels of p53 expression in primary lesions and nodal metastasis. Two patients have only p53 expression in nodal metastasis.
The impact of p53 as a prognostic marker was investigated in 82 SSCP patients undergoing penile amputation and bilateral inguinal lymphadenectomy [2]. Immunoreactivity of p53 was studied with other clinicopathological parameters, and HPV DNA was detected by PCR using generic primers. Nuclear accumulation of p53 was detected in 34 of 82 samples (41.5 %). Clinical lymph node N stage, lymphatic and venous embolization by neoplastic cells, and p53 positivity were significantly associated with lymph node metastasis. Multivariate analysis revealed that only lymphatic embolization and p53 positivity were independent factors for lymph node metastasis. Patients with negative p53 had significantly better 5- and 10-year overall survival than those in whom tumors stained positive for p53. When tumors were p53 positive and HPV DNA positive, overall survival was worse.
The prognostic significance of p53, Ki-67, E-cadherin, and Matrix metalloproteases-9 (MMP-9) was evaluated in SSCP tumors of 73 Chinese patients who have penile amputation and regional lymphadenectomy [42]. The expression of molecular markers was determined by immunohistochemistry. By multivariate analysis, tumor embolization and the expression of p53 were independent predictors of metastasis. In stage T1 tumors, high expression of p53 was significantly associated with metastasis and poor survival.
Telomerase Activity
A telomere is the “cap” at the end of chromosomes. It is composed of repetitive DNA sequences and specialized proteins that protect the end of the chromosome from degradation of genes near the ends of chromosomes. The loss of telomeric DNA sequences that occur in each cell division rules the process of cell aging or senescence. Very short telomeres trigger the cell death process, so that they act as a “molecular clock” that determines the lifetime of a cell. Cell immortalization depends on stopping telomeric DNA degradation by the reactivation of telomerase that adds DNA sequence repeats (“TTAGGG” in all vertebrates) to the 3’ end of DNA strands in the telomere.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree