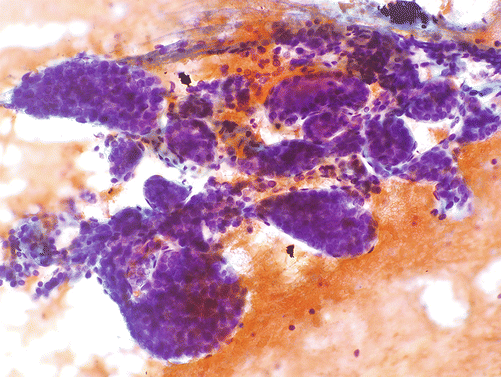
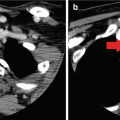
Fig. 4.1
Overturning the aspiration needle and tapping the material on the slide can often facilitate removal of partially clotted specimen in the needle hub
Once a small amount of aspirated material is placed on a glass slide, smear preparations need to be made very quickly. Although there are some variations on the exact technique, making direct smears is a simple and straightforward process. One glass slide (face up) containing an aliquot of specimen is pressed against another glass slide (face down), and slight pressure is placed on the “glass sandwich” to spread the material across the surface area of both glass slides (Fig. 4.2). At this point, the slides are slid across each other, and the specimen is ideally “smeared” evenly across both slide surfaces. In this manner, two slides are obtained per aliquot of specimen. In most instances, one slide will be left to “air-dry,” and the remaining slide will be placed in an alcohol-based fixative.


Fig. 4.2
Aspirated specimen is “smeared” over two glass slides by applying gentle pressure while sliding slides away from each other
Most cytopathologists have been trained to evaluate both types of “direct smear” specimens. The air-dried slide is usually stained with a Giemsa-based stain. The advantages of this type of preparation are visualization of extracellular colloid, a feature which is extremely important for evaluation of the presence or absence of neoplasia. The second type of preparation, prepared from the alcohol-fixed slide, allows for better interpretation of individual cell nuclear detail. These two types of slide preparation emphasize different features of the cytologic sample and provide complementary information for cytologic evaluation.
There are no recommendations on the number of needle “passes” that are required to obtain a diagnostic specimen. Specimen “adequacy” is a function of several factors including the nature of the thyroid lesion to be sampled and operator expertise. Any additional material remaining after preparation of “direct smears” may be submitted to the laboratory as a “rinse” of the needle contents. The rinses are often prepared as additional components of the patients’ case materials. The latter may include a “cell block” which often contains microscopic fragments of intact tissue. Alternatively, the needle may be “rinsed” into a liquid-based medium for further preparation. Of note, liquid-based media slides (without direct smears) perform poorly vs. conventional preparations [1–3].
Rapid on-site evaluation (ROSE) of aspirated material involves interpretation of a portion of the specimen during the procedure. This usually involves a cytotechnologist or cytopathologist be present at the time of the procedure with appropriate resources (microscope, stains, slides, etc.) to render an immediate interpretation. With thyroid FNAs, this is usually performed to assess the sample for adequacy. Studies have shown that the addition of ROSE to FNA of the thyroid is likely to reduce the rate of inadequate specimens [4, 5]. This results in increased efficiency and cost savings for the patient. Unfortunately, the performance of ROSE involves a substantial input of resources, both time and effort, upon the part of the laboratory. Each practice should evaluate the pros and cons of utilizing ROSE.
Terminology for Reporting Thyroid Fine-Needle Aspiration
Fine-needle aspiration of the thyroid has become a mainstay in screening and triaging individuals with thyroid nodules. Some estimates show that less than 5% of all nodules are malignant, and FNA has led to a significant decrease in the number of operations performed for benign thyroid nodules [6, 7].
Prior to widespread cytologic screening, less than 15% of surgically resected nodules were malignant [7]. Since implementation of a widespread screening program, the percentage of malignancy in resected nodules is now estimated at 50% [8]. It is clear that FNA plays an important role in management of thyroid nodules and has led to extensive cost savings and a reduction in unnecessary overtreatment.
The current system for reporting FNA of the thyroid, The Bethesda System (TBS) for Reporting Thyroid Cytopathology, came from a 2007 consensus conference of expert pathologists, radiologists, surgeons, and endocrinologists. The goal of the group was to provide a system for reporting thyroid cytopathology results that was clear, concise, and unambiguous [9]. An additional goal was to promote uniformity in practice and reporting of thyroid FNAs. There were also efforts to link each individual category with defined risks of malignancy and to propose guidelines for further management.
TBS has since been implemented and widely adopted in the USA. The current iteration of TBS includes six discreet diagnostic categories. The categories include a “nondiagnostic” or unsatisfactory for diagnosis (Class I) as well as an additional five categories associated with variable levels of concern and implied risk of malignancy for any given thyroid sample. While “risk” of malignancy is estimated for each category, it is important to know that true sensitivity, specificity, and positive and negative predictable values are difficult to determine as only a subset of thyroid nodules will be removed for histologic examination, currently considered the “gold standard” for cytologic accuracy. These features are summarized in Table 4.1. Characteristics of each individual class are discussed in more detail in the following descriptions.
Table 4.1
Pathologic hallmarks of PTC
Nuclear features | Architectural features |
|---|---|
Nuclear grooves “coffee bean,” “crushed ping-pong ball” | Papillae |
Intranuclear cytoplasmic inclusions (INCIs) or pseudoinclusions | Psammoma bodies |
Elongated nuclei, powdery chromatin | Nuclear crowding |
“Orphan Annie eyes”—only seen on surgical pathology specimens | May be encapsulated or infiltrative—this cannot be assessed on cytology |
Class I: Nondiagnostic or Unsatisfactory
First and foremost, specimens need to contain a minimum number of cells in order to be deemed “satisfactory” for evaluation. Under the current Bethesda guidelines, the cellularity requirement is at least six groups of ten cells. A specimen may also be deemed unsatisfactory if there is excessive air-drying artifact, if the cells are largely obscured with blood, or if the smears are overly thick. Exceptions to the numerical requirement include a number of different scenarios:
A specimen with abundant colloid can be presumed to be benign despite the cell requirement. In this instance, the presumed diagnosis is a colloid nodule.
When a specific diagnosis can be rendered, such as lymphocytic thyroiditis, the specimen is by definition adequate, even if the number of identifiable follicular cells is less than the adequacy threshold.
If there is any significant cytologic atypia in an otherwise hypocellular aspirate, a Class III or higher diagnosis may be rendered.
In some instances, cytopathologists are comfortable interpreting a specimen consisting entirely of macrophages (cyst contents) as benign. This practice is not uniformly accepted by all laboratories.
The recommended management of a nondiagnostic specimen is repeat aspiration. Repeat aspiration should be performed with ultrasound guidance for nodules that are small and/or difficult to detect by palpation alone. The percentage of cases that are deemed unsatisfactory will depend on numerous factors including operator expertise, utilization of ROSE, specimen preparation, and individual cytopathologists’ comfort with low cellularity samples. The benchmark for percentage of unsatisfactory specimens is below 10% of all thyroid samples [10].
Class II: Benign
The majority of thyroid nodules (70%) are reported as benign [10]. These lesions characteristically have abundant colloid and small, normally sized groups of thyroid follicular epithelium (Fig. 4.3). The histologic correlate to these findings is usually a benign follicular nodule or a hyperplastic nodule, often in a background of multinodular goiter. The false-negative rate associated with a benign diagnosis very low, estimated at 1–3% [11]. Clinical follow-up of the patient is recommended for patients with a Class II diagnosis.
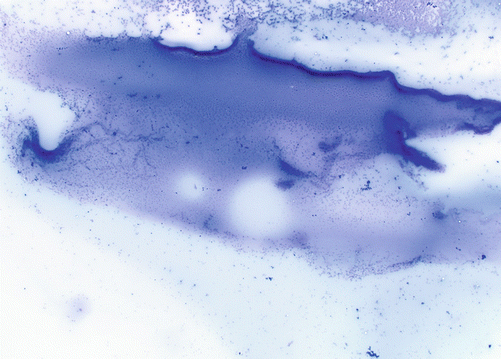

Fig. 4.3
Colloid is best visualized on air-dried Giemsa-based preparations. It can be variably thick or “watery” but appears as a thin film in the background of fine-needle aspirate smears (Diff-Quick, mag × 10)
Class III: Atypia of Undetermined Significance or Follicular Lesion of Undetermined Significance
This particular diagnosis is often frustrating for both the patient and clinician because of the use of the vague word “atypia” without any further clarification. There is also an implied “low” but nevertheless present risk of malignancy. There are many different strategies for follow-up of a Class III diagnosis ranging from repeat FNA to surgical excision. Reasons for an “atypical” diagnosis often include one or more of the following factors:
There is a prominent population of thyroid follicular epithelium in an otherwise sparsely cellular aspirate.
There is a predominance of Hürthle cells in a sparse aspirate with scant colloid (without other features to suggest lymphocytic (Hashimoto) thyroiditis).
There are numerous Hürthle cells in the aspirate yet the clinical or imaging features suggest a benign thyroid nodule.
There are focal features of atypia (nuclear grooves or enlarged overlapping cells) in aspirate in patients with known or suspected Hashimoto thyroiditis.
Focal cytologic atypia is identified in association with cyst lining cells.
When the Bethesda system of reporting approved the inclusion of the category of atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), they legitimize the presence of an equivocal diagnostic category for cytopathologists to utilize to communicate their uncertainty about the potential for malignancy. As suspected, the overall number of cases (previously termed “atypical”) increased from an estimated 3% of total diagnoses to approximately 7% in a single institution study [12]. The 7% threshold has become the accepted benchmark for percentage of specimens reported under TBS; however, there is significant variation among different laboratories and individual cytopathologists [13–15]. In an effort to contain potential overutilization of the AUS/FLUS category, there have been attempts to develop performance measures (specifically ratios of AS/FLUS rates to “malignant” rates) as guidelines or benchmarks for individual laboratories [16]. This type of ratio guideline is currently well accepted and utilized as a performance measure for cervical cancer screening reporting [17].
Current estimates of a true malignancy associated with a Class III AUS/FLUS diagnosis are relatively low (5–15%) but nevertheless real [18–20]. The NCI consensus treatment algorithm for these patients is repeat FNA after an appropriate interval [8, 20]. A significant number of these patients will receive a subsequent diagnosis of a Class III or higher diagnosis on repeat aspiration. At this point, appropriate approaches include biopsy with molecular testing (see Chap. 5) or more aggressive management strategies. Despite the recommended guidelines, many patients with an AUS/FLUS diagnosis will proceed directly to surgery. While there may be numerous extenuating factors to justify this strategy (patient desire, worrisome imaging features, etc.), this approach has been deemed less cost-effective than recommended strategies for overall patient management [21].
Class IV: Follicular Neoplasm or Suspicious for Follicular Neoplasm
This reporting category is largely confined to aspirates comprised of either follicular epithelium arranged in “microfollicles” or with an abundance of Hürthle cells. The latter is a morphologically distinctive cell type and should be reported as such (Fig. 4.4). When a microfollicular arrangement of slightly enlarged but otherwise normal-appearing epithelial cells is present, the lesion is most likely to correspond to one of the following: an adenomatoid nodule, follicular adenoma, or carcinoma. Likewise, a hypercellular aspirate comprised of Hürthle cells can correspond to a hyperplastic proliferations within a background benign disease (Hashimoto thyroiditis or multinodular goiter), Hürthle cell adenoma, and carcinoma.
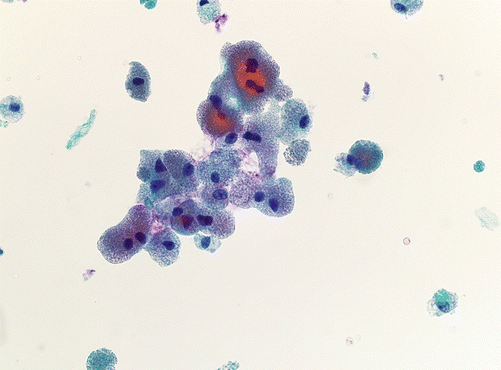

Fig. 4.4
Hürthle cells can appear in a variety of circumstances. They appear as epithelioid or polygonal cells with abundant granular cytoplasm (Pap stain, mag × 200)
While some have opined that this category overlaps substantially with the previous category of “FLUS” and is thus redundant, there are numerous reasons for separating this lesion from the former. Firstly, the specific nomenclature (“lesion” vs. “neoplasm”) is meant to convey a higher diagnostic concern for possible malignancy. Secondly, “follicular neoplasms” include both the benign adenoma and the malignant counterpart, follicular carcinoma. In aspirates, these two lesions are cytologically indistinguishable; the only way to separate one from another is complete removal of the nodule with careful examination of the capsule of the lesion. Lastly, the terms “lesion” linked to category III and “neoplasm” linked to category IV have been linked to “low-risk” and “high-risk” nodules from a clinical standpoint [22].
Class IV diagnoses (follicular neoplasm (FN) and suspicious for follicular neoplasm (SFN)) are more likely to correspond to significant thyroid pathology than any of the previously three categories. In this instance, up to 35% will correspond to benign lesions, an estimated 15–30% corresponds to true malignancies (carcinoma), and the remainder likely represents adenomas [19, 23–25]. The corresponding management recommendation for a Class IV diagnosis is either molecular testing or surgical lobectomy.
Class V: Suspicious for Malignancy
Class V specimens generally display some but not all features of cytologic malignancy. For papillary carcinoma these include cellular enlargement and overlapping, arrangement in irregular papillary-like groups, and nuclear groves or pseudoinclusions. “Suspicious” samples often contain less colloid than benign aspirates. The term suspicious for malignancy will also include samples suspected to be from medullary carcinoma, anaplastic carcinoma, metastases, and lymphoma. Cytopathologists are strongly encouraged to report which particular subtype of malignancy is suspected.
One of the most common reasons for a “suspicious” diagnosis (vs. malignant) is aspirations of either a very small or largely cystic papillary carcinoma. The latter is particularly difficult to diagnose with confidence as the aspirates display lower cellularity and usually have a secondary population of cyst lining cells. Cyst lining cells may predominate to an extent that the neoplastic cells are a small minority of the aspirated sample. In addition, hyalinizing trabecular adenoma (HTA) will often show numerous cytologic features that overlap substantially with papillary carcinoma. An aspirate of HTA can be misread as either a Class IV or V papillary carcinoma and represents a well-known pitfall in thyroid cytopathology diagnosis [26, 27].
Malignancy rates associated with a Class V diagnosis are reported to range from 60 to 75% [9]. The most commonly associated malignancies include papillary carcinoma, the follicular variant of papillary carcinoma, and follicular neoplasms.
Class IV: Malignant
This category is used when unequivocal features of malignancy are identified. The most commonly diagnosed malignancy in this category is papillary carcinoma. Other types of cancers identifiable in aspirates include medullary and anaplastic carcinomas, as well as metastases and lymphomas. The positive predictive value for this category is 97–99%. The recommended treatment is thyroidectomy (for primary malignancies).
Core-Needle Biopsy
Core-needle biopsy (CNB) of the thyroid has evolved as an alternative to fine-needle aspiration (FNA) for sampling thyroid lesions. Studies comparing CNB and FNA have largely shown that sensitivity and specificity are similar for both techniques; however, CNB is less likely to yield a nondiagnostic or inadequate sample [28–30]. The one exception to this trend was in a cohort of pediatric patients, for whom the nondiagnostic rate was higher than comparable values for FNA (in adult patients) [31]. For the present, CNB is being used largely as an alternative to repeat FNA (rFNA) in patients with an initial indeterminate diagnosis (atypia of uncertain significance (AUS) or follicular lesion of undetermined significance (FLUS)). Several studies have noted that a combination of FNA and CNB (performed simultaneously) increases both sensitivity and specificity over either technique used alone [30, 32, 33]. In this setting, CNB appears to be useful and can be helpful in discriminating patients who should be referred to surgery versus those that should be managed more conservatively [32, 34–37].
CNB of the thyroid has some definite advantages over FNA in selected cases. In primary thyroid lymphoma, CNB appears to be more efficacious in establishing a definitive diagnosis than FNA [38–40]. The superiority of CNB in the diagnosis of lymphoma may be related to the need for additional material for ancillary studies: particularly flow cytometry and/or immunohistochemistry. In contrast, CNB does not perform well in the “follicular neoplasm” category [41, 42]. Because both FNA and CNB cannot adequately assess the completeness and integrity of the capsule of these lesions, neither of these modalities is useful for discriminating the benign follicular adenoma from a follicular carcinoma.
The Role of Frozen Section Examination in Thyroid Disease
The role of frozen section in the management of thyroid disease has changed since the fine-needle aspiration cytology has become widely used. Before preoperative FNAs became common, frozen section was often used to guide operative management of patients with thyroid nodules. All patients with “cold” nodules on scintigraphy were referred for surgery, and intraoperative frozen section was performed to determine the necessary extent of surgery (benign lesions underwent a lobectomy, malignant lesions underwent total thyroidectomy). Now, FNA is the preferred triage test, and the role of frozen section is much more limited, although it continues to be requested in some settings. The primary reason for obtaining a frozen section is to allow a total thyroidectomy as the initial procedure and avoid a second (completion) procedure.
Overall, frozen section of the thyroid is reported to be highly specific (approximately 95%) but relatively insensitive (approximately 66%), with a positive predictive value of approximately 95%. Fine-needle aspiration has similar specificity, sensitivity, and positive predictive value [43]. However, sensitivity and specificity of both fine-needle aspiration and frozen section vary dramatically depending on the type of lesion sampled (follicular vs. non-follicular), and unfortunately most of the lesions which are problematic by fine-needle aspiration are also problematic by frozen section [44]. Neither test has 100% positive predictive value. However, there are some situations where frozen section may add additional information to a preoperative FNA that may help guide surgical management.
Given the accuracy of the diagnosis of “positive for malignancy” (Bethesda category 6) and “negative for malignancy” (Bethesda category 2), several studies have concluded that frozen section does not significantly add useful information that would aid in planning the scope of surgery for patients with these FNA diagnoses, although this is not uniformly accepted [45, 46]. An FNA categorized as positive for malignancy has a specificity rate above 90%, so those that do not support frozen section in this situation argue that even if a frozen section was benign, it would likely be considered a false negative [45, 46]. In a study of cases with a benign preoperative FNA, 16% of these were shown to be malignant on final diagnosis, but only 29% of these were detected by frozen section. Overall, in cases with a benign preoperative FNA diagnosis, frozen section rarely provided a definitive diagnosis of malignancy that converted a planned surgical procedure to a total thyroidectomy [46].
There does appear to be a role for frozen sections with preoperative FNA diagnoses of “suspicious for malignancy” (Bethesda category 5), particular for those interpreted as suspicious for papillary thyroid carcinoma. This category has a risk of malignancy of 60–75% [9]. In many cases, frozen section provides a definitive cancer diagnosis to allow a total thyroidectomy as an initial procedure [45–47]. If the frozen section is benign or deferred, the procedure might be limited to a lobectomy. In the minority of cases with benign or deferred frozen sections and permanent pathology showing a malignancy (sometimes related to sampling error of the frozen section, sometimes for subtle malignancies such as follicular variant of papillary carcinoma which can be challenging on frozen section), then the patient might need a completion procedure, but the number of these can be minimized by frozen section. The goal of frozen section in this category is to allow as many as cases as possible to undergo an initial total thyroidectomy, if necessary, without overtreating a benign nodule.
As the treatment paradigm has shifted toward more liberal use of lobectomy as definitive treatment of differentiated cancers, an important consideration in the use of frozen section is whether or not the result will change management. If lobectomy will be considered adequate for the tumor in question, then clearly the addition of frozen section will not be useful. Some features of the tumor that might indicate the need for more aggressive treatment, such as microscopic extrathyroidal invasion or vascular invasion, would not be expected to be reliably detected on frozen section.
The most problematic management areas are cases with preoperative FNAs of atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS, Bethesda category 3) and follicular neoplasm/suspicious for follicular neoplasm (FN/SFN, Bethesda category 4). These will be considered separately. AUS/FLUS is estimated to have a malignancy rate of 5–15%, though this rate varies by institution [9], and the recommended management is to repeat the FNA in 3–6 months. Molecular testing is also an option for lesions with this diagnosis. However, some patients and surgeons choose surgical management for nodules which show AUS/FLUS on FNA (particular for repeated AUS/FLUS results), and in these patients, frozen section may be a useful adjunct to clinical findings in choosing appropriate surgery. Some have advocated incorporating ultrasound findings in choosing which patients for which to request frozen sections (with nodules >1 cm, increased vascularity, calcifications, irregular cystic areas, and irregular borders being indications for frozen section) [48].
Follicular neoplasm/suspicious for follicular neoplasm is a particularly difficult area for both cytology and frozen sections. As described previously, this category is used for lesions composed of microfollicles, which may be seen in both benign follicular adenomas and malignant follicular carcinomas, and the distinction between the two is made by identifying capsular and/or vascular invasion. Invasive properties of the capsule cannot be identified by cytology, and thus the diagnosis of malignancy in a follicular neoplasm by FNA is essentially impossible. Capsular or vascular invasion may be very focal, making sampling by frozen section problematic. A meta-analysis comparing fine-needle aspirations and frozen sections of thyroid nodules found that for follicular neoplasms, frozen section had a 21% sensitivity but a 99% specificity for the diagnosis of malignancy in a total of 23 studies covering 2531 cases [43]. Whether this low rate of definitive diagnosis is worth the cost of the frozen section remains a topic of intense debate. Additionally, surgeons need to understand that a follicular neoplasm with a negative or deferred frozen section may be shown to be a follicular carcinoma with complete submission of the capsule for histologic evaluation, something that is not possible at frozen section [47].
In summary, there appears to be little to no role of frozen section in cases that have cytologic diagnoses of benign and malignant. Frozen sections may be useful in cases with cytologic diagnoses of AUS/FLUS (particular persistent AUS/FLUS and/or those with suspicious ultrasound findings). The consensus of the literature is that the category suspicious for malignancy can benefit from a frozen section (particularly in cases with a preoperative diagnosis of suspicious for papillary carcinoma). Frozen section for follicular neoplasms can, in rare instances, add additional information to the preoperative cytologic diagnosis.
Surgical Pathology of Differentiated Thyroid Cancers
Differentiated thyroid tumors arise from thyroid follicular cell origin and fall generally into two broad categories, papillary carcinoma and follicular carcinoma, with some morphologic areas of overlap between the two entities [49–51].
Papillary Thyroid Carcinoma
Papillary thyroid carcinoma (PTC) is the most common thyroid malignancy and presents in a wide range of sizes, from nodules appreciated on physical examination to so-called microcarcinomas that are often found incidentally in thyroids removed for benign disease. In its classical form, PTC is characterized by papillary architecture and distinct nuclear features that may appreciated on fine-needle aspiration (FNA) cytology [9, 50] Table 4.1.
FNA
PTC is characterized by nuclear contour irregularities, which manifest on cytology as nuclear grooves and pseudoinclusions. The “pseudo” in pseudoinclusions, or intranuclear cytoplasmic inclusions (INCIs), refers to the fact that these are not truly inclusions within the nuclei, but rather are pockets formed by the irregular nuclear membrane surrounding cytoplasm which remains contiguous with the rest of the cell’s cytoplasm. Other nuclear features include powdery chromatin and nuclear elongation [9, 10, 52].
Architectural features may include papillary structures or sheets and nuclear crowding (Fig. 4.5). Psammoma bodies, concentric calcified structures, are not always present in PTC, but are highly suggestive of PTC as they are only rarely seen in benign entities. These psammoma bodies may correspond to the microcalcifications seen on ultrasound [53].
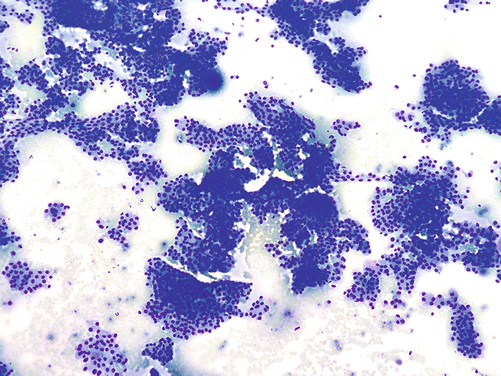

Fig. 4.5
Papillary thyroid carcinoma, fine-needle aspiration cytology, Diff-Quik, 4×: cellular sample with scant colloid, papillary architecture, and overlapping nuclei
Regarding FNA of PTC, the diagnostic nuclear features are best appreciated on alcohol-fixed material, which better preserves nuclear detail (Fig. 4.6). While a well-preserved and adequately cellular FNA sample of a classical PTC allows for ready diagnosis, conversely, a sample with scant cellularity, lack of alcohol-fixed material, or obscuring factors may limit the ability of the cytopathologist to render a definitive diagnosis of malignancy.
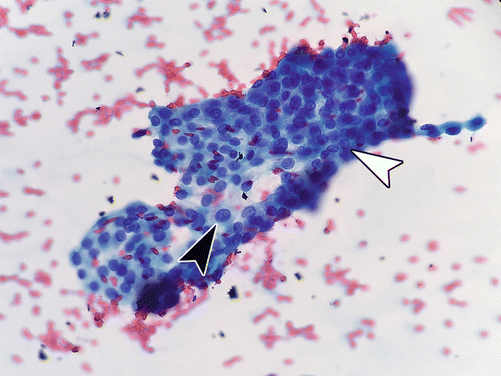

Fig. 4.6
Papillary thyroid carcinoma, fine-needle aspiration cytology, alcohol-fixed Papanicolaou stain, 40×: nuclei demonstrating classic features of pseudoinclusions (arrowhead), elongation, and grooves (open arrow) [note to publisher: this figure will need to be large]
Gross Examination
At the time of gross examination, papillary thyroid carcinomas are variable in their appearance and may be firm and white in color (Fig. 4.7), in contrast to the gelatinous consistency and red color of normal thyroid. Depending on the subtype, they may be infiltrative or encapsulated.
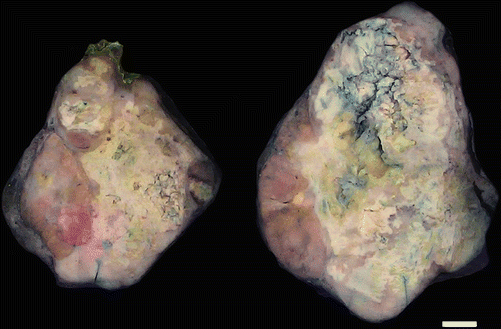

Fig. 4.7
Gross photograph of thyroid carcinoma: a heterogenous pale process effaces normal thyroid parenchyma. Necrosis is grossly evident. On microscopic examination, this lesion was an oncocytic carcinoma with areas of anaplastic carcinoma corresponding to the areas with necrosis
Microscopic Features
The features seen on cytology are mirrored in the papillary architecture and nuclear features seen on H&E stained sections (Figs. 4.8, 4.9, and 4.10). In addition, nuclei may demonstrate chromatin clearing (an artifact of formalin fixation) resulting in an “Orphan Annie eye” appearance after the comic of yesteryear. While the classical type of PTC is the most common, comprising approximately 40% of PTC, there are a number of additional subtypes. These subtypes are not only important in that they can pose diagnostic challenges for the surgical pathologist but also in that certain ones may carry prognostic significance [49, 51, 54].
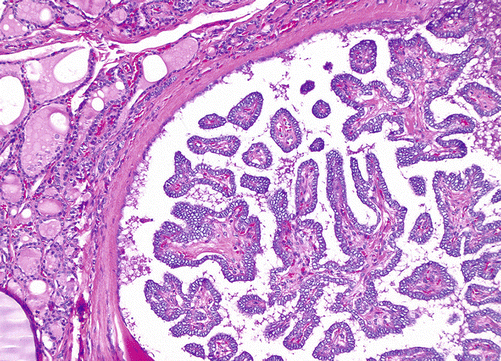
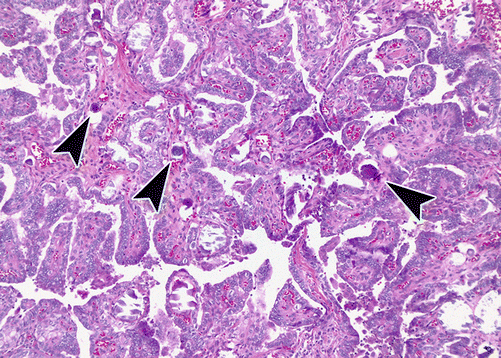
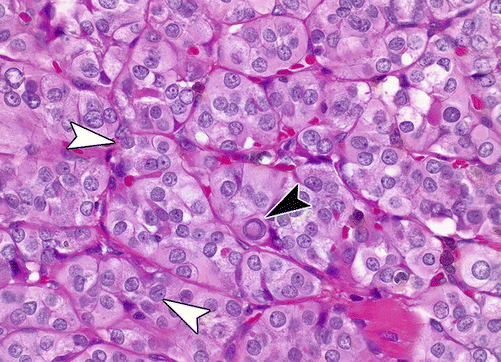

Fig. 4.8
Papillary thyroid carcinoma, H&E, 10×. Well-demarcated tumor demonstrating papillary architecture and nuclear clearing (compare to normal thyroid on left of image)

Fig. 4.9
Papillary thyroid carcinoma, H&E, 10×. Papillary architecture with numerous psammoma bodies, concentrically laminated basophilic structures (arrowheads). These may correspond to the “microcalcifications” classically seen on ultrasound

Fig. 4.10
Papillary thyroid carcinoma, 40×. A prominent nuclear pseudoinclusion is seen (arrowhead) as well as nuclear elongation and grooves (open arrows)
PTC Variants
Papillary microcarcinoma is defined as a carcinoma that is 1 cm in size or less and is found incidentally [49] (Fig. 4.11). These tumors are common and may be found in thyroids removed for benign reasons such as compressive symptoms [55]. The majority of these small tumors have indolent clinical behavior, but a small proportion of these tumors will still have worrisome characteristics such as extrathyroidal extension, lymph node metastases, and recurrence [56–58].
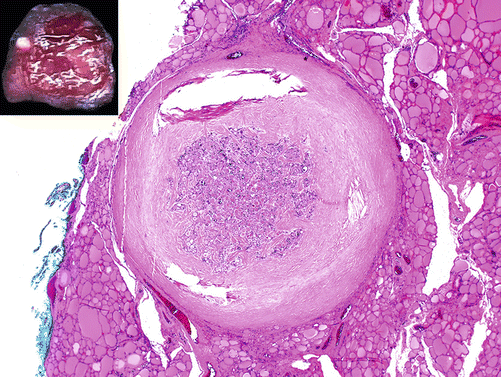

Fig. 4.11
Papillary microcarcinoma, H&E, 2×. A well-circumscribed nodule with a thick fibrous capsule. Inset: Gross photo of this papillary microcarcinoma: a well-demarcated white fibrotic nodule is seen
Follicular variant of PTC (FVPTC) is the second most common variant of PTC characterized by a predominant follicular pattern, lack of papillae, and the presence of nuclear features of PTC (Fig. 4.12). This variant poses challenges diagnostically, as it is a heterogenous and controversial group of tumors that shares features with PTC, follicular carcinomas, and follicular adenoma [59–61]. There is considerable interobserver variability in the diagnosis of these tumors, which may be due to attempting to impose a binary diagnostic classification (cancer/not cancer) upon a biological continuum of mutation accumulation leading from benign follicular adenoma to malignant follicular lesion [23, 24, 47, 51, 60, 62–65]. Due to the follicular architecture and generally less well-developed nuclear features of PTC, these tumors are often not able to be definitively diagnosed on FNA, rather yielding FNA diagnoses of AUS/FLUS, SFN, FN, or suspicious for malignancy [65, 66]. Genetically, these tumors tend to be characterized by mutations (RAS, BRAF K601E, Pax8/PParɣ) which are more similar to those seen in follicular tumors than in classical PTC [67–71].
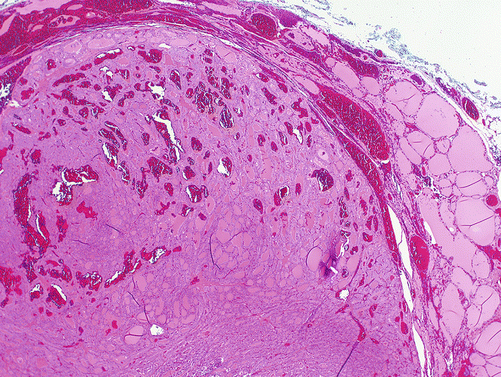

Fig. 4.12
Follicular variant of papillary thyroid carcinoma, H&E, 2×. A well-circumscribed tumor with follicular architecture
The encapsulated follicular variant of PTC is a subgroup characterized by clearly encapsulated tumors with follicular architecture and often does not show evidence of invasion into adjacent structures or vessels. These noninvasive encapsulated FVPTCs have recently been characterized by an effort of a consortium of endocrine pathologists as having indolent behavior, and it has been suggested that these represent a separate entity to be termed noninvasive follicular thyroid neoplasm with papillary-like nuclear features rather than true malignancies [72]. As all of these tumors were in the past treated as PTC, time and additional follow-up data will be needed to determine whether these truly represent a benign entity; however, the initial reports are promising [73, 74].
In contrast to the above variants, tall cell variant is a variant which carries potentially a worse prognosis than classical PTC. In order to qualify as tall cell variant, >50% of the tumor cells must show tall cell morphology, where the tumor cell height is at least 3× width [49]. These tumors often present in older patients and at a higher tumor stage based on extrathyroidal extension and metastases [75]. Perhaps due to these findings, these tumors are associated with a worse 5-year survival than classical PTC [76]. In addition, these tumors are often found as a differentiated component in anaplastic and poorly differentiated thyroid carcinomas, suggesting they are more likely to undergo dedifferentiation [77]. It has been suggested that even a minor tall-cell component less than 50% may also carry worse prognosis, though further study is required to determine whether this is truly the case [78, 79].
There are numerous other rare variants including columnar cell, solid, Warthin-like, clear cell (Fig. 4.13), cribriform-morular, oxyphilic (Hürthle cell), diffuse sclerosing, and variants with hobnail features or fasciitis-like stroma. The prognostic significance of these other variants is generally less well established, likely due to their rarity.
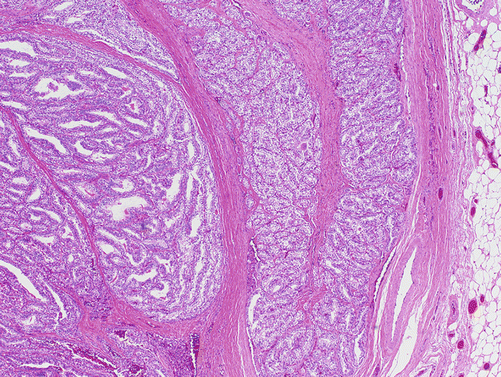

Fig. 4.13
Clear cell variant of PTC, H&E, 4×. Thyroid tumor with diffuse cytoplasmic clearing
Follicular Thyroid Carcinoma
Follicular thyroid carcinoma (FTC) is the second most common thyroid carcinoma, after PTC. These tumors are characterized by a follicular pattern and lack of nuclear features of PTC and are defined by evidence of capsular or vascular invasion. Capsular and lymphovascular invasion are architectural features that require histologic examination and cannot be assessed on cytology. For this reason, FNA as a modality cannot reliably make the distinction between follicular adenoma and follicular carcinoma [25]. The FNA findings for both follicular adenomas and carcinomas are generally in the spectrum of FLUS/SFN/follicular neoplasm [12, 14, 19, 24, 64, 65] (Fig. 4.14).